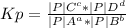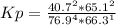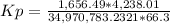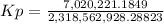Hydrogen chloride and oxygen react to form chlorine and water, like this:
4 HCl(g) + O2(g) → 2 Cl2(g) + 2 H2O(g)
Also, a chemist finds that at a certain temperature the equilibrium mixture of hydrogen chloride, oxygen, chlorine, and water has the following composition:
COMPOUND Pressure at equilibrium
HCl 76.9 atm
O2 66.3 atm
Cl2 40.7 atm
H2O 65.1 atm
Calculate the value of the equilibrium constant Kp for this reaction. Round your answer to 2 significant digits.

Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 23:00
Achef makes salad dressing by mixing oil, vinegar, and spices, as shown. which type of matter is the salad dressing?
Answers: 1

Chemistry, 22.06.2019 01:40
C3h8o3 - glycerol major species present when dissolved in water
Answers: 2


Chemistry, 22.06.2019 11:00
Which statement correctly identifies the scientific question and describes why the question is scientific? question 1 refers to the supernatural.question 2 reflects a moral or social value.question 3 refers to something that can be measured.question 4 reflects a question that can’t be observed.
Answers: 1
You know the right answer?
Hydrogen chloride and oxygen react to form chlorine and water, like this:
4 HCl(g) + O2...
4 HCl(g) + O2...
Questions

Biology, 16.07.2019 23:50

Physics, 16.07.2019 23:50

English, 16.07.2019 23:50



History, 16.07.2019 23:50

English, 16.07.2019 23:50





English, 16.07.2019 23:50








Mathematics, 16.07.2019 23:50