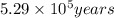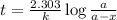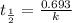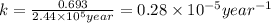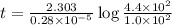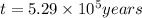Radioactive plutonium-239 (t½ = 2.44 x 105 yr) is used in nuclear reactors and atomic bombs. If there are 4.4 x 102 g of the isotope in a small atomic bomb, how long will it take (in yr) for the substance to decay to 1.0 x 102 g, too small an amount for an effective bomb? This radioactive decay follows first order kinetics.

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 11:00
As air becomes more dense, (select all that apply) o. air weighs less o. gas molecules are closer together o. air is colder o. air weighs more o. gas molecules are further apart o. air is hotter
Answers: 3

Chemistry, 22.06.2019 12:00
Explain what happens at the saturation point when adding salt to water at room temperature.
Answers: 1

Chemistry, 22.06.2019 18:00
Chlorophyll a had the molecular formula c55h72mgn4o5 how many atoms are in this molecule
Answers: 2

Chemistry, 23.06.2019 02:30
Which statement best describes the liquid state of matter? a. it has definite shape but indefinite volume. b. it has definite shape and definite volume. c. it has indefinite shape and indefinite volume. d. it has indefinite shape but definite volume.
Answers: 1
You know the right answer?
Radioactive plutonium-239 (t½ = 2.44 x 105 yr) is used in nuclear reactors and atomic bombs. If ther...
Questions


Advanced Placement (AP), 16.10.2020 05:01




Advanced Placement (AP), 16.10.2020 05:01




English, 16.10.2020 05:01

Chemistry, 16.10.2020 05:01

English, 16.10.2020 05:01

Mathematics, 16.10.2020 05:01

Social Studies, 16.10.2020 05:01

History, 16.10.2020 05:01


Mathematics, 16.10.2020 05:01



Biology, 16.10.2020 05:01

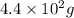 to
to  is
is