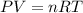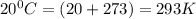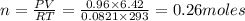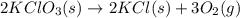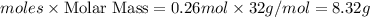Oxygen gas can be prepared by heating potassium chlorate according to the following equation:
<...

Oxygen gas can be prepared by heating potassium chlorate according to the following equation:
2KClO3(s) → 2KCl(s) + 3O2(g)
The product gas, O2, is collected over water at a temperature of 20 °C and a pressure of 747 mm Hg. If the wet O2 gas formed occupies a volume of 6.42 L, the number of grams of O2 formed is g. The vapor pressure of water is 17.5 mm Hg at 20 °C.

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 01:50
Ase your answer to this question on the information below.hydrocarbons and fissionable nuclei are among the sources used for the production of energy in the united states. a chemical reaction produces much less energy than a nuclear reaction per mole of reactant.the balanced chemical equation below represents the reaction of one molecule of a hydrocarbon with two molecules of oxygen.chemical equation: ch4 + 2o2 → co2 + 2h2o + 1.48 × 10−18 jthe nuclear equation below represents one of the many possible reactions for one fissionable nucleus. in this equation, x represents a missing product.nuclear equation: write an isotopic notation for the missing product represented by x in the nuclear equation.
Answers: 1


Chemistry, 22.06.2019 09:40
In the lab, ammonia was mixed with water to form ammonium hydroxide. what is/are the reactant(s)? o water and ammonia o ammonia o ammonium hydroxide need
Answers: 2

Chemistry, 22.06.2019 13:00
One of the hopes for solving the world's energy problem is to make use of the fusion reaction 21h +31h --> 42he + 10n + energy how much energy is released when 1 mol of deuterium is fused with 1 mol of tritium according to the above reaction? the masses of the atoms and the neutrons are as follows: 21h = 2.0140 amu 31h = 3.01605 amu 42he = 4.002603 amu 10n = 1.008665 amu. the speed of light is 2.9979 x 108 m/s.
Answers: 1
You know the right answer?
Questions

Mathematics, 31.08.2019 14:30





History, 31.08.2019 14:30

History, 31.08.2019 14:30

Chemistry, 31.08.2019 14:30

History, 31.08.2019 14:30






Social Studies, 31.08.2019 14:30

History, 31.08.2019 14:30


Mathematics, 31.08.2019 14:30

English, 31.08.2019 14:30

Social Studies, 31.08.2019 14:30

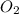 formed is 8.32 g
formed is 8.32 g