Chemistry, 27.03.2020 18:09 aaronolivera200161
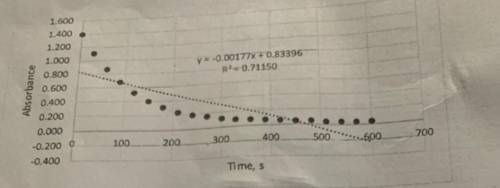
Determine the value of k' from the slope. These graphs were plotted in terms of absorbance, but the rate constant should be in terms of concentration. Beer-Lambert's law and the literature value for the molar absorptivity constant (87,000 M^-1cm^-1 can be used to convert the slope from units of absorbance/s to units of concentration/s. This value is the pseudo rate constant, k'.

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 04:00
Asolution contains 225 g of sodium chloride, nacl, dissolved in enough water to make a 0.25 l of solution. what is the molarity of the solution?
Answers: 2

Chemistry, 22.06.2019 06:00
Which of the following did jj thompson discover about atoms? a)an atom has an internal structure. b) atoms are tiny indivisible particles. c)electrons orbit the nucleus of an atom. d) the nucleus of an atom contains protons and neutrons.
Answers: 2

Chemistry, 22.06.2019 14:10
13. a covalent bond between two atoms is likely to be polar if: a. one of the atoms is much more electronegative than the other. b. the two atoms are equally electronegative. c. the two atoms are of the same element. d. the bond is part of a tetrahedrally shaped molecule. e. one atom is an anion.
Answers: 1

Chemistry, 22.06.2019 15:30
Count the number of each type of atom in the equation below, and then balance the equation. write in the numbers of atoms and coefficients. add a 1 if there should be no coefficient. cs2(l) + o2(g) → co2(g) + so2(g) c [ ] s [ ] o > c [ ] s [ ] o [ ] cs2(l) + [ ] o2(g) > [ ] co2(g) + [ ] so2(g)
Answers: 3
You know the right answer?
Determine the value of k' from the slope. These graphs were plotted in terms of absorbance, but the...
Questions

Mathematics, 16.04.2021 23:30

Mathematics, 16.04.2021 23:30

Mathematics, 16.04.2021 23:30



Mathematics, 16.04.2021 23:30

Mathematics, 16.04.2021 23:30

Biology, 16.04.2021 23:30

Mathematics, 16.04.2021 23:30

Mathematics, 16.04.2021 23:30




Mathematics, 16.04.2021 23:30

English, 16.04.2021 23:30

Mathematics, 16.04.2021 23:30

Mathematics, 16.04.2021 23:30

Mathematics, 16.04.2021 23:30

Mathematics, 16.04.2021 23:30

Mathematics, 16.04.2021 23:30