
Chemistry, 27.03.2020 16:45 janahiac09
What is the change in freezing point (delta T) of an aqueous solution that is 0.082 molality AlCl3?
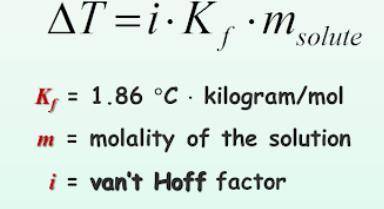

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 02:30
When you perform this reaction, what could remain at the end of the reaction? check all that apply. excess reactant aqueous copper chloride excess reactant aluminum oxygen product solid copper carbon dioxide product aqueous aluminum chloride water
Answers: 2

Chemistry, 22.06.2019 11:00
The number to the right of an element's symbol (ex. c-12) identifies the of an isotope.
Answers: 1

Chemistry, 22.06.2019 11:50
The chemical bond connecting one nucleotide with the next one along the nucleic acid chain is called a
Answers: 3

Chemistry, 22.06.2019 17:10
Acalorimeter is to be calibrated: 51.203 g of water at 55.2 degree c is added to a calorimeter containing 49.783 g of water at 23.5c. after stirring and waiting for the system to equilibrate, the final temperature reached is 37.6 degree c. specific heat capacity of water (s = 4.18 j/g∙degree c). calculate the calorimeter constant. (smδt)warm water = -[(smδt)cold water + (calorimeterδtcold water)]
Answers: 2
You know the right answer?
What is the change in freezing point (delta T) of an aqueous solution that is 0.082 molality AlCl3?<...
Questions

Mathematics, 16.02.2021 23:30

Biology, 16.02.2021 23:30





Mathematics, 16.02.2021 23:30

Mathematics, 16.02.2021 23:30

Health, 16.02.2021 23:30



Mathematics, 16.02.2021 23:30

History, 16.02.2021 23:30

Spanish, 16.02.2021 23:30

History, 16.02.2021 23:30

Chemistry, 16.02.2021 23:30


Mathematics, 16.02.2021 23:30

Mathematics, 16.02.2021 23:30



