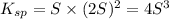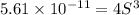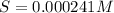Chemistry, 26.03.2020 23:46 tinasidell1972
Mg(OH)2 is a sparingly soluble compound, in this case a base, with a solubility product, Ksp, of 5.61×10−11. It is used to control the pH and provide nutrients in the biological (microbial) treatment of municipal wastewater streams. Based on the given value of the Ksp, what is the molar solubility of Mg(OH)2 in pure H2O?

Answers: 3
Another question on Chemistry

Chemistry, 23.06.2019 02:00
What can be done to make a solid solute dissolve faster in a liquid solvent?
Answers: 1


Chemistry, 23.06.2019 08:10
Time remaining 58: 10 an atom that has 84 protons and 86 neutrons undergoes a reaction. at the end of the reaction, it has 82 protons and 84 neutrons. what happened to the atom? it accepted radiation in a chemical reaction it donated neutrons to another atom in a chemical reaction it emitted an alpha particle in a nuclear reaction. it accepted protons in a nuclear reaction. mark this and retum save and exit next submit
Answers: 3

Chemistry, 23.06.2019 10:00
An uncovered pot of water lies out in the sun. which statements correctly describe what happens at the surface of the liquid water? 1. the vapor pressure remains constant regardless of the water temperature. 2. the vapor pressure is produced by water molecules that have evaporated. 3. the vapor pressure increases as the sun heats the water in the pot. 4. evaporation stops once the vapor pressure reaches a certain point. 5. evaporation and condensation both occur on the liquid’s surface.
Answers: 3
You know the right answer?
Mg(OH)2 is a sparingly soluble compound, in this case a base, with a solubility product, Ksp, of 5.6...
Questions




Business, 01.10.2019 14:00


Mathematics, 01.10.2019 14:00



Biology, 01.10.2019 14:00


History, 01.10.2019 14:00

Social Studies, 01.10.2019 14:00


Advanced Placement (AP), 01.10.2019 14:00

Mathematics, 01.10.2019 14:00




Mathematics, 01.10.2019 14:00

Mathematics, 01.10.2019 14:00

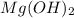 in pure water.
in pure water.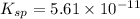
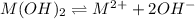
![K_{sp}=[M^{2+}][OH^-]^2](/tpl/images/0566/2963/a461b.png)