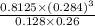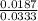Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 19:30
If the root word engage means “to connect with something,” what does the word disengage mean in the following sentence? he disengaged the gears by stepping on the clutch pedal.a.added more engine powerb.activated a connection to the pedalc.stalled the engined.released a connection to the pedal
Answers: 1

Chemistry, 22.06.2019 05:30
Which of the following two events occur to create a sea breeze? select all that apply. warm air rises on the ocean and moves toward the land to cool warm air rises on land and moves toward the ocean to cool cool air moves from the ocean to be warmed by the land cool air moves from the land to be warmed by the ocean
Answers: 3

Chemistry, 22.06.2019 09:30
Melissa is interested in her family tree and how her family has changed over its many generations. melissa probably more closely resembles
Answers: 2

Chemistry, 22.06.2019 18:10
Measurements that have similar values are: a. usually accurate b. sometimes accurate c. always accurate d. never accurate
Answers: 1
You know the right answer?
In a study of the conversion of methane to other fuels, a chemical engineer mixes gaseous CH4 and H2...
Questions


History, 18.06.2021 17:30

English, 18.06.2021 17:30




Biology, 18.06.2021 17:30

English, 18.06.2021 17:30


Geography, 18.06.2021 17:30


Mathematics, 18.06.2021 17:30

Social Studies, 18.06.2021 17:30

Mathematics, 18.06.2021 17:30

Mathematics, 18.06.2021 17:30

English, 18.06.2021 17:30




![[H_{2}O]](/tpl/images/0566/0391/04475.png) at equilibrium is 0.561 M.
at equilibrium is 0.561 M.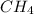 = 0.041 mol,
= 0.041 mol,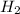 = 0.091 mol
= 0.091 mol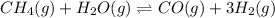
![\frac{[CO][H_{2}]^{3}}{[CH_{4}][H_{2}O]}](/tpl/images/0566/0391/7f129.png) ...... (1)
...... (1)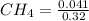 = 0.128 M,
= 0.128 M,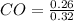 = 0.8125 M,
= 0.8125 M, 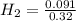 = 0.284 M
= 0.284 M![[H_{2}O] = \frac{[CO][H_{2}]^{3}}{[CH_{4}] \times K}](/tpl/images/0566/0391/7448a.png)