
Chemistry, 26.03.2020 21:12 torquishag
Consider the following reaction in which all reactants and products are gases. 1.00 mol of A and 2.00 mol of B are placed in a 5.0-liter container. After equilibrium has been established, 0.50 mol of D is present in the container. Calculate the equilibrium constant, Kc, for the reaction.

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 11:20
Which of the following contributes to the structural rigidity of cellulose? adjacent glucose polymers are stabilized by hydrogen bonding. glucose residues are joined by (α1→4) linkages. cellulose is a highly branched molecule. the conformation of the glucose polymer is a coiled structure.
Answers: 2

Chemistry, 22.06.2019 22:00
If a solution contains 3 moles/liter of sodium chloride (nacl, made of sodium ions and chloride ions), what is the osmolarity of this solution
Answers: 3

Chemistry, 23.06.2019 06:50
The student repeated the experiment using a higher concentration of acid. the same volume of acid and the same mass of magnesium ribbon were used. what volume of hydrogen gas would have been produced after 60 seconds?
Answers: 1

Chemistry, 23.06.2019 10:30
Me soon im confused much mass would a mole of hydrogen molecules contain? recall that hydrogen is diatomic. g/mol
Answers: 1
You know the right answer?
Consider the following reaction in which all reactants and products are gases. 1.00 mol of A and 2.0...
Questions







Social Studies, 12.07.2019 20:40

English, 12.07.2019 20:40









Physics, 12.07.2019 20:40


Mathematics, 12.07.2019 20:40

![K_{c} = \frac{[D]}{[A][B]}](/tpl/images/0565/8107/95178.png) =
= 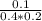 =1.25
=1.25

