Chemistry, 25.03.2020 05:38 codyfore141
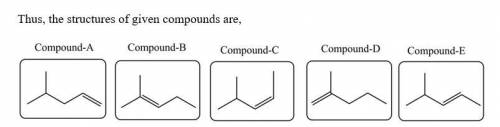
Five isomeric alkenes A–E each undergo catalytic hydrogenation to give 2-methylpentane. The IR spectra of these five alkenes have the following key absorptions (in cm–1): Compound A: 912 (s), 994 (s), 1643 (s), 3077 (m) Compound B: 833 (s), 1667 (w), 3050 (weak shoulder on C–H absorption) Compound C: 714 (s), 1665 (w), 3010 (m) Compound D: 885 (s), 1650 (m), 3086 (m) Compound E: 967 (s), no absorption 1600–1700, 3040 (m) The alkene structures are given below. Identify each compound.

Answers: 3
Another question on Chemistry


Chemistry, 22.06.2019 00:30
Jessica is traveling from miami, florida, to chicago, illinois. using the map, tell one way the land will change during the second half of her trip.
Answers: 1

Chemistry, 22.06.2019 03:30
At a temperature of 393 k, the temperature of a sample of nitrogen is 1.07 atm what will the pressure be at a temperature of 478 k
Answers: 1

You know the right answer?
Five isomeric alkenes A–E each undergo catalytic hydrogenation to give 2-methylpentane. The IR spect...
Questions


Mathematics, 10.04.2021 04:30


Mathematics, 10.04.2021 04:30

Geography, 10.04.2021 04:30


Mathematics, 10.04.2021 04:30


Mathematics, 10.04.2021 04:30






History, 10.04.2021 04:30

History, 10.04.2021 04:30