
Chemistry, 25.03.2020 05:51 kokokakahi
A mixture initially contains A, B, and C in the following concentrations: [A] = 0.600 M , [B] = 1.30 M , and [C] = 0.500 M . The following reaction occurs and equilibrium is established: A+2B⇌C At equilibrium, [A] = 0.410 M and [C] = 0.690 M . Calculate the value of the equilibrium constant, Kc.

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 02:40
Achange in the number of neutrons in an atom will change an blank . when the number of protons changes in an atom, a new element will form.
Answers: 2

Chemistry, 22.06.2019 15:30
Using the first volume and temperature reading on the table as v1 and t1, solve for the unknown values in the table below. remember to use the rules of significant figures when entering your numeric response.
Answers: 2

Chemistry, 22.06.2019 16:50
Which of the following is an indication that a substance has undergone a chemical change? a. no new product has been formed. b. the color of the substance has not changed. c. the original constitute has not changed. d. the molecular structure has changed.
Answers: 1

Chemistry, 22.06.2019 21:30
While in europe, if you drive 125 km per day, how much money would you spend on gas in one week if gas costs 1.10 euros per liter and your car’s gas mileage is 32.0 mi/gal? assume that 1 euro=1.26 dollars
Answers: 2
You know the right answer?
A mixture initially contains A, B, and C in the following concentrations: [A] = 0.600 M , [B] = 1.30...
Questions



Mathematics, 04.10.2021 19:10

English, 04.10.2021 19:10


Mathematics, 04.10.2021 19:10



History, 04.10.2021 19:10

Biology, 04.10.2021 19:10

English, 04.10.2021 19:10

Social Studies, 04.10.2021 19:10



Chemistry, 04.10.2021 19:10

English, 04.10.2021 19:10

SAT, 04.10.2021 19:10

Biology, 04.10.2021 19:10

History, 04.10.2021 19:10

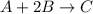
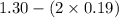
![K_{eq} = \frac{[C]}{[A][B]^{2}}](/tpl/images/0562/6350/666b5.png)
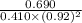
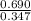
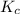 is 1.988.
is 1.988.

