
Predict whether the equilibria
I) CO2(g) + 2 NH3(g) =
CO(NH2)2(s) + H2O(g),
AH° = -90 kJ
II) Ni(s) + 4 CO(g) = Ni(CO)4(8),
AH° = -161 kJ
will shift toward products or reactants with a
temperature increase.
1. I shifts toward reactants and II shifts
toward products.
2. Both I and II shift toward products.
3. Unable to determine
4. Both I and II shift toward reactants.
5. I shifts toward products and II shifts to-
ward reactants.

Answers: 3
Another question on Chemistry

Chemistry, 21.06.2019 13:00
When light strikes a plane mirror, images form in locations where light does not actually reach. it only appears to the observer as though the light were coming from this position. what type of image is formed?
Answers: 2

Chemistry, 21.06.2019 20:00
When a comet collides with earth, it adds material to our planet and causes great damage. therefore, a collision like this is a a. destructive force b. constructive force c. geologic process and event d. constructive and destructive force
Answers: 1

Chemistry, 21.06.2019 21:40
During trial 2, what allowed you to determine that aluminum was the limiting reactant? check all that apply. all of the copper dissolved. all of the aluminum dissolved. the solution turned clear. the number of grams of copper(ii) chloride used in the reaction was greater than the number of grams of aluminum. the molar ratio of copper(ii) chloride to aluminum was greater than 3: 2, the equation’s molar ratio.
Answers: 2

Chemistry, 22.06.2019 09:00
Acrystal that absorvd water from air is (blank)a. aqueousb. homogenousc. hygroscopicd. efflorescent
Answers: 1
You know the right answer?
Predict whether the equilibria
I) CO2(g) + 2 NH3(g) =
CO(NH2)2(s) + H2O(g),
AH° =...
I) CO2(g) + 2 NH3(g) =
CO(NH2)2(s) + H2O(g),
AH° =...
Questions

Business, 07.05.2021 14:00

Physics, 07.05.2021 14:00

Mathematics, 07.05.2021 14:00

Physics, 07.05.2021 14:00

Physics, 07.05.2021 14:00


Chemistry, 07.05.2021 14:00

Physics, 07.05.2021 14:00


World Languages, 07.05.2021 14:00


Chemistry, 07.05.2021 14:00

Mathematics, 07.05.2021 14:00


Mathematics, 07.05.2021 14:00

English, 07.05.2021 14:00

Mathematics, 07.05.2021 14:00



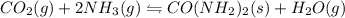 ,ΔH° = -90 kJ
,ΔH° = -90 kJ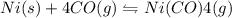 ,ΔH° = -161 kJ
,ΔH° = -161 kJ

