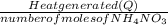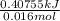Chemistry, 25.03.2020 02:03 AkramMasoud
Calculate the change in enthalpy for the reaction. (Use 1.0 g/mL as the density of the solution and 4.18 J/(g⋅∘C) as the specific heat capacity.) Express the enthalpy change in kilojoules per mole to two significant figures. ΔHrxn = nothing kJ/mol

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 04:30
How many grams of co(g) are there in 74.5 ml of the gas at 0.933 atm and 30o c?
Answers: 1

Chemistry, 22.06.2019 06:00
One of the few xenon compounds that form is cesium xenon heptafluoride (csxef7). how many moles of csxef7 can be produced from the reaction of 13.0 mol cesium fluoride with 12.5 mol xenon hexafluoride? csf(s) + xef6(s) csxef7(s)
Answers: 1

Chemistry, 23.06.2019 03:20
High-pressure liquid chromatography (hplc) is a method used in chemistry and biochemistry to purify chemical substances. the pressures used in this procedure range from around 500 kilopascals (500,000 pa) to about 60,000 kpa (60,000,000 pa). it is often convenient to know the pressure in torr. if an hplc procedure is running at a pressure of 1.03×108 pa , what is its running pressure in torr?
Answers: 3

Chemistry, 23.06.2019 15:30
Sodium chloride can be made as follows: 2na + cl2 ? 2nacl i calculate the maximum amount of nacl possible if 2.3 g of sodium was reacted with excess chlorine. show all your workings.
Answers: 3
You know the right answer?
Calculate the change in enthalpy for the reaction. (Use 1.0 g/mL as the density of the solution and...
Questions


Mathematics, 16.06.2020 05:57


History, 16.06.2020 05:57




Mathematics, 16.06.2020 05:57


Mathematics, 16.06.2020 05:57


English, 16.06.2020 05:57


Mathematics, 16.06.2020 05:57

Mathematics, 16.06.2020 05:57


Law, 16.06.2020 05:57

Mathematics, 16.06.2020 05:57


Mathematics, 16.06.2020 05:57

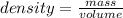
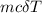
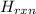 =
=