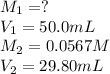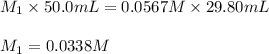Chemistry, 25.03.2020 00:28 itzdulceee
Acetic acid (CH3CO2H) is the principal component in the vinegar. What is the molarity of an acetic acid solution if a titration of 50.00 mL of the acetic acid solution requires 29.80 mL of 0.0567 M NaOH to reach the equivalence point?

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 14:50
Given the following information: mass of proton = 1.00728 amu mass of neutron = 1.00866 amu mass of electron = 5.486 × 10^-4 amu speed of light = 2.9979 × 10^8 m/s calculate the nuclear binding energy (absolute value) of 3li^6. which has an atomic mass of 6.015126 amu. j/mol.
Answers: 2

Chemistry, 22.06.2019 15:00
‘which reaction would most likely require the use of an inert electrode?
Answers: 1


Chemistry, 22.06.2019 19:40
What is the wavelength of a 3*10^12 hz infrared wave a 3*10^20m b 1* 10^4m c 3*10^-3m d 1*10^-4 m
Answers: 1
You know the right answer?
Acetic acid (CH3CO2H) is the principal component in the vinegar. What is the molarity of an acetic a...
Questions



English, 27.10.2020 23:30





Mathematics, 27.10.2020 23:30

Mathematics, 27.10.2020 23:30

Mathematics, 27.10.2020 23:30

Chemistry, 27.10.2020 23:30





Mathematics, 27.10.2020 23:30

Mathematics, 27.10.2020 23:30


Social Studies, 27.10.2020 23:30

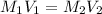
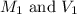 are the molarity and volume of acetic acid.
are the molarity and volume of acetic acid.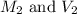 are the molarity and volume of NaOH.
are the molarity and volume of NaOH.