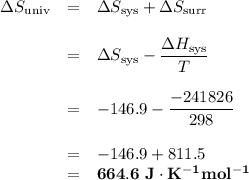Calculate the ΔH, ΔS and ΔSuniverse for this reaction.

Chemistry, 24.03.2020 23:01 Cartucho1978
½O2(g) + H2(g) ⇌ H2O(g)
Calculate the ΔH, ΔS and ΔSuniverse for this reaction.
Determine the spontaneity of the reaction.

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 07:30
The volume of helium in a blimp is 6.28 x 10^9 millimeters. the density of helium in the blimp is .1786 kilogram/meter^3. find the mass of the helium in the blimp.
Answers: 1

Chemistry, 22.06.2019 20:10
What would happen to a volleyball left outside in the winter? o o o o a. it would expand. b. it would lose air. c. it would shrink. d. it would explode.
Answers: 2

Chemistry, 23.06.2019 01:00
Which description best characterization the motion of particles in a solid
Answers: 1

Chemistry, 23.06.2019 01:30
The solubility of barium nitrate is 9.02 g/100 g h2o at 20°c. a 15.2 g sample of barium nitrate is added to 200.0 g of water at 20°c. is the solution saturated, unsaturated, or supersaturated? a. unsaturated b. saturated c. supersaturated
Answers: 1
You know the right answer?
½O2(g) + H2(g) ⇌ H2O(g)
Calculate the ΔH, ΔS and ΔSuniverse for this reaction.
Calculate the ΔH, ΔS and ΔSuniverse for this reaction.
Questions


Mathematics, 30.06.2019 06:00



History, 30.06.2019 06:00



Mathematics, 30.06.2019 06:00

Business, 30.06.2019 06:00



Biology, 30.06.2019 06:00

Mathematics, 30.06.2019 06:00

Mathematics, 30.06.2019 06:00


Biology, 30.06.2019 06:00


History, 30.06.2019 06:00