
Chemistry, 24.03.2020 20:14 nehakarakkattu
Given that a chlorine-oxygen bond has an enthalpy of 243 kJ/mol , an oxygen-oxygen bond has an enthalpy of 498 kJ/mol , and the standard enthalpy of formation of ClO2 102.5 kJ/mol , calculate the value for the enthalpy of formation per mole of ClO(g). What is the value for the enthalpy of formation per mole of ClO(g)? Enter your answer numerically, in terms of kJ, and to three significant figures.

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 03:30
Asample of ammonia reacts with oxygen as shown. 4nh3(g) + 5o2(g) 4no(g) + 6h2o(g) what is the limiting reactant if 4.0 g of nh3 react with 8.0 g of oxygen? o2 because it produces only 0.20 mol of no. nh3 because it produces only 0.20 mol of no. o2 because it produces two times less no than nh3. nh3 because it produces three times more no than o2.
Answers: 3

Chemistry, 22.06.2019 12:00
What is the lowest number energy level where a d sublevel is found
Answers: 1

Chemistry, 22.06.2019 22:00
Choose all the answers that apply. fluorine (f) has an atomic number of 9 and an atomic weight of 18.99. fluorine has a. 9 protons b. 10 neutrons c. 18 electrons d. an atomic mass of 19 e. at least one isotope
Answers: 1

Chemistry, 23.06.2019 02:00
Scientists are often interested in knowing the molar heat of combustion – the heat released during the combustion of one mole of a substance. use the periodic table to find molar masses. how many moles of ethanol are present in the sample?
Answers: 2
You know the right answer?
Given that a chlorine-oxygen bond has an enthalpy of 243 kJ/mol , an oxygen-oxygen bond has an entha...
Questions

Social Studies, 23.10.2020 19:10

English, 23.10.2020 19:10

History, 23.10.2020 19:10

Mathematics, 23.10.2020 19:10

English, 23.10.2020 19:10


Mathematics, 23.10.2020 19:10


Mathematics, 23.10.2020 19:10





History, 23.10.2020 19:10

Biology, 23.10.2020 19:10

Mathematics, 23.10.2020 19:10


Mathematics, 23.10.2020 19:10


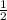
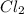 (g) +
(g) + 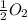 (g) → ClO(g)
(g) → ClO(g) 

