
Chemistry, 24.03.2020 19:49 Lovelybunny321
Methylamine, CH3NH2, is a weak base that reacts according to the reaction CH3NH2 + H2O <--> CH3NH3+ + OH- The value of the ionization constant, Kb, is 5.25 x 10 –4. Methylamine reacts to form salts such as methylammonium nitrate, (CH3NH3+)(NO3-). a. Calculate the hydroxide ion concentration, [OH-] of a 0.125 molar aqueous solution of methylamine.

Answers: 2
Another question on Chemistry

Chemistry, 21.06.2019 16:20
Consider the two electron arrangements for neutral atoms a and b. are atoms a and b the same element? a - 1s2, 2s2, 2p6, 3s1 b - 1s2, 2s2, 2p6, 5s1
Answers: 3

Chemistry, 22.06.2019 10:30
Which describes fat? a: a carbohydrate that produces energy b: a nucleic acid that directs cell function c: a lipid that stores energy d: a protein that speeds up a chemical reaction
Answers: 1

Chemistry, 22.06.2019 10:50
8) a mixture of he, ne and ar has a pressure of 7.85 atm. if the ne has a mole fraction of 0.47 and 8) ar has a mole fraction of 0.23, what is the pressure of he? a) 4.2 atm b) 3.7 atm c) 5.5 atm d) 2.4 atm e) 1.8 atm
Answers: 1

You know the right answer?
Methylamine, CH3NH2, is a weak base that reacts according to the reaction CH3NH2 + H2O <--> CH...
Questions



Business, 05.08.2020 14:01


Mathematics, 05.08.2020 14:01

Mathematics, 05.08.2020 14:01

Spanish, 05.08.2020 14:01

Biology, 05.08.2020 14:01

Computers and Technology, 05.08.2020 14:01

Geography, 05.08.2020 14:01



Biology, 05.08.2020 14:01

English, 05.08.2020 14:01

Biology, 05.08.2020 14:01

English, 05.08.2020 14:01




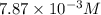
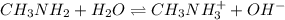
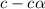
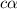
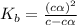
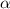 = ?
= ?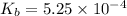
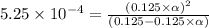
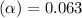
![[OH^-]=c\times \alpha](/tpl/images/0561/1862/0ea5e.png)
![[OH^-]=0.125\times 0.063=7.87\times 10^{-3}M](/tpl/images/0561/1862/08078.png)


