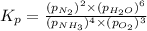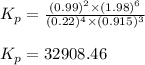Ammonia has been studied as an alternative "clean" fuel for internal combustion engines, since its reaction with oxygen produces only nitrogen and water vapor, and in the liquid form it is easily transported. An industrial chemist studying this reaction fills a flask with of ammonia gas and of oxygen gas, and when the mixture has come to equilibrium measures the partial pressure of nitrogen gas to be . Calculate the pressure equilibrium constant for the combustion of ammonia at the final temperature of the mixture.

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 12:30
Consider the four elements above. which one of these elements will combine with oxygen in a 1: 1 ratio?
Answers: 3

Chemistry, 22.06.2019 17:00
Reduction is a reaction which results in a in electrons and a in positive charge of the atom or ion 1) a- loss 1) b- gain 2) a-increase 2) b-decrease
Answers: 1

Chemistry, 22.06.2019 17:50
You exhale co2 which is produced during cellular respiration. co2 combines with the water in your blood's plasma to make up one half of the body's most important buffer pair, carbonic acid. the more physical activity you engage in, the more co2 your body is producing. you can see this by putting some of the cabbage indicator in a glass and then blowing bubbles into it through a straw. can you see a change in the color of the indicator?
Answers: 2

Chemistry, 22.06.2019 21:20
40dm3 of gas at 760 torr are heated from 5°c to 50°c what is the new volume
Answers: 3
You know the right answer?
Ammonia has been studied as an alternative "clean" fuel for internal combustion engines, since its r...
Questions



Mathematics, 05.05.2020 08:23



Mathematics, 05.05.2020 08:23



Social Studies, 05.05.2020 08:23




Biology, 05.05.2020 08:23




Advanced Placement (AP), 05.05.2020 08:23



English, 05.05.2020 08:23

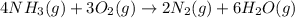
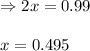
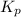 for above equation follows:
for above equation follows: