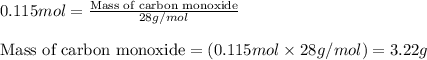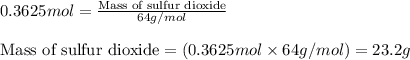Chemistry, 24.03.2020 16:34 micahatwood03
For the following reaction, 11.6 grams of sulfur are allowed to react with 23.5 grams of carbon monoxide . sulfur(s) + carbon monoxide(g) sulfur dioxide(g) + carbon(s) What is the maximum amount of sulfur dioxide that can be formed? grams What is the FORMULA for the limiting reagent? CO What amount of the excess reagent remains after the reaction is complete? grams

Answers: 3
Another question on Chemistry

Chemistry, 21.06.2019 21:30
An alcohol thermometer makes use of alcohol's changing in order to measure temperature. as the temperature goes up, the alcohol contained in the thermometer increases in volume, filling more of the thermometer's tube.
Answers: 3

Chemistry, 22.06.2019 11:30
For each of the following compounds, decide whether the compound's solubility in aqueous solution changes with ph. if the solubility does change, pick the ph at which you'd expect the highest solubility. you'll find ksp data in the aleks data tab. compounds does solubility change with ph
Answers: 3

Chemistry, 22.06.2019 22:30
Is the idea of spontaneous generation supported by redi's experiment? justify your answer in 2-3 sentences?
Answers: 1

Chemistry, 23.06.2019 06:00
Nthis lab, you will do experiments to identify types of changes. using the question format you learned (shown above), write an investigative question that you can answer by doing these experiments
Answers: 3
You know the right answer?
For the following reaction, 11.6 grams of sulfur are allowed to react with 23.5 grams of carbon mono...
Questions

Mathematics, 16.10.2020 23:01


Computers and Technology, 16.10.2020 23:01




Mathematics, 16.10.2020 23:01


Mathematics, 16.10.2020 23:01

Chemistry, 16.10.2020 23:01

SAT, 16.10.2020 23:01



Mathematics, 16.10.2020 23:01


Business, 16.10.2020 23:01

English, 16.10.2020 23:01

Mathematics, 16.10.2020 23:01


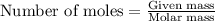 .....(1)
.....(1)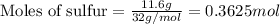
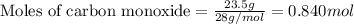
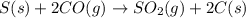
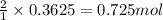 of carbon monoxide
of carbon monoxide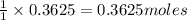 of sulfur dioxide
of sulfur dioxide