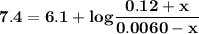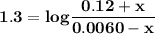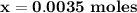Blood is buffered by carbonic acid and the bicarbonate ion. Normal blood plasma is 0.024 Min HCO?3 and 0.0012 M H2CO3 (pKa1 for H2CO3 at body temperature is 6.1).
A. What is the pH of blood plasma?
I got 7.4 for pH which is the correct answer
B. If the volume of blood in a normal adult is 5.0 L, what mass of HCl could be neutralized by the buffering system in blood before the pH fell below 7.0 (which would result in death)?
C. Given the volume from part B, what mass of NaOHcould be neutralized before the pH rose above 7.8?
Express your answer using two significant

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 06:30
If 1.8 l of water is added to 2.5l of a 7.0 molarity koh solution, what is the molarity of the new solution
Answers: 1


Chemistry, 22.06.2019 13:00
Adepositional also feature that forms where a stream enters a lake or an ocean is a
Answers: 2

Chemistry, 22.06.2019 15:20
Which description best characterizes the motion of particles in a solid?
Answers: 2
You know the right answer?
Blood is buffered by carbonic acid and the bicarbonate ion. Normal blood plasma is 0.024 Min HCO?3 a...
Questions




















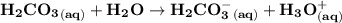
![\mathbf{pH = pKa_1 + log \dfrac{[HCO_3^-]}{[H_2CO_3]}}](/tpl/images/0560/4723/d4625.png)
![\mathbf{pH =6.1 + log \dfrac{[0.024]}{[0.0012]}}](/tpl/images/0560/4723/a7134.png)
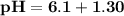
![\mathbf{pH = pKa_1+ log \dfrac{[ salt]}{[acid] }}](/tpl/images/0560/4723/dbb85.png)
![\mathbf{7 =6.1+ log \dfrac{[ 0.024 \times 5 -x]}{[0.0012 \times 5 +x] }}](/tpl/images/0560/4723/43579.png)
![\mathbf{0.9= log \dfrac{[ 0.024 \times 5 -x]}{[0.0012\times 5 +x] }}](/tpl/images/0560/4723/2dcb7.png)
![\mathbf{10^{0.9}= \dfrac{[ 0.024 \times 5 -x]}{[0.0012 \times 5 +x] }}](/tpl/images/0560/4723/8672b.png)
![\mathbf{7.94 = \dfrac{[ 0.024 \times 5 -x]}{[0.0012 \times 5 +x] }}](/tpl/images/0560/4723/b837c.png)
![\mathbf{7.94 = \dfrac{[ 0.12-x]}{[0.006 +x] }}](/tpl/images/0560/4723/81a5e.png)
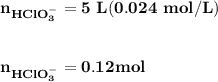
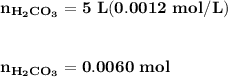
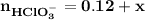
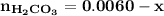
![\mathbf{pH = pKa_1+ log \dfrac{[ HCO_3^-]}{[H_2CO_3] }}](/tpl/images/0560/4723/f8866.png)