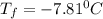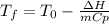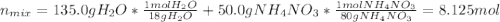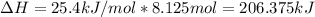Chemistry, 24.03.2020 02:26 ellaemtagedeane
Some instant cold packs contain ammonium nitrate and a separate pouch of water. When the pack is activated by squeezing to break the water pouch, the ammonium nitrate dissolves in water and the pack gets cold. The heat of solution for ammonium nitrate is 25.4 kJ/mol.
a) Is the dissolution of ammonium nitrate endothermic or exothermic?
b) A cold pack contains 135.0 g of water and 50.0 g of ammonium nitrate. What will be the final temperature of the activated cold pack, if the initial temperature is 25.0 degree C? (Assume that the specific heat of the solution is the same as that for water, 4.184 J/g degree C and no heat is lost).

Answers: 3
Another question on Chemistry


Chemistry, 22.06.2019 10:10
Stage in which a star’s outer layers have started to cool and grow outward?
Answers: 3

Chemistry, 22.06.2019 15:30
Count the number of each type of atom in the equation below, and then balance the equation. write in the numbers of atoms and coefficients. add a 1 if there should be no coefficient. cs2(l) + o2(g) → co2(g) + so2(g) c [ ] s [ ] o > c [ ] s [ ] o [ ] cs2(l) + [ ] o2(g) > [ ] co2(g) + [ ] so2(g)
Answers: 3

Chemistry, 22.06.2019 21:30
In science class richard learns that a substance has a boiling point of 230 fahrenheit his teacher ask him to convert this temperature to degrees celsius what is the boiling point of his substance in degrees celsius
Answers: 3
You know the right answer?
Some instant cold packs contain ammonium nitrate and a separate pouch of water. When the pack is act...
Questions

Mathematics, 13.02.2022 19:50

English, 13.02.2022 19:50

Mathematics, 13.02.2022 19:50


Chemistry, 13.02.2022 19:50

Biology, 13.02.2022 19:50

History, 13.02.2022 19:50

English, 13.02.2022 19:50

Mathematics, 13.02.2022 19:50



English, 13.02.2022 20:00

Mathematics, 13.02.2022 20:00


Mathematics, 13.02.2022 20:00

Mathematics, 13.02.2022 20:00


Mathematics, 13.02.2022 20:00


Mathematics, 13.02.2022 20:00