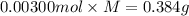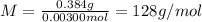A sample of a monoprotic acid (HA) weighing 0.384 g is dissolved in water and the solution is titrated with aqueous NaOH. If 30.0 mL of 0.100 M NaOH is required to reach the equivalence point, what is the molar mass of HA?
(a) 211 g/mol
(b) 128 g/mol
(c) 81.0 g/mol
(d) 37.0 g/mol
(e) 20.3 g/mol

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 10:00
3. how much energy in joules is required to evaporate .0005 kg of liquid ammonia to vapor at the same temperature? 4. how much energy ( in megajoules ) is given up by .75 kg of water at 0c when it freezes to form ice at 0c? 5. explain how heat works between and at critical temperatures?
Answers: 2

Chemistry, 22.06.2019 10:00
Americium-241 undergoes fission to produce three neutrons per fission event. if a neutron-absorbing material is mixed in with this sample so that the rate of neutron production drops down to 1.8 neutrons per fission event, which will be effective at achieving a critical mass? check all that apply. remove a deflective shield surrounding the sample. remove absorbent material mixed in with the sample. compress the sample of americium-241.
Answers: 1

Chemistry, 22.06.2019 10:10
Stage in which a star’s outer layers have started to cool and grow outward?
Answers: 3

Chemistry, 22.06.2019 17:10
Increasing the substrate concentration in an enzymatic reaction could overcome which of the following? a) the need for a coenzymeb) allosteric inhibitionc) competitive inhibitiond) insufficient cofactors
Answers: 1
You know the right answer?
A sample of a monoprotic acid (HA) weighing 0.384 g is dissolved in water and the solution is titrat...
Questions

Mathematics, 05.02.2021 07:40


Mathematics, 05.02.2021 07:40




History, 05.02.2021 07:40

Mathematics, 05.02.2021 07:40

Biology, 05.02.2021 07:40




Mathematics, 05.02.2021 07:40


English, 05.02.2021 07:40



English, 05.02.2021 07:40


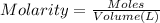
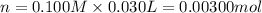
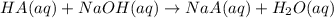
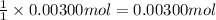 of HA
of HA