
Chemistry, 23.03.2020 20:35 mariahrpoulin9630
Sodium acetate spontaneously crystallizes out of a supersaturated solution upon standing or upon the addition of a seed crystal. Which of the following is true for the thermodynamic quantities of this system for thisprocess?(a)ΔS < 0J/K, ΔH < 0 J(b)ΔS < 0J/K, ΔH > 0 J(c)ΔS > 0J/K, ΔH < 0J(d)ΔS > 0J/K, ΔH > 0J

Answers: 3
Another question on Chemistry

Chemistry, 21.06.2019 20:50
Choose all that apply. when creating a graph, you should: determine the x- and y- variables label the scale on the x- and y- axes plot the data points draw a line of best fit to represent the data trend
Answers: 1

Chemistry, 22.06.2019 12:00
Consider the following reaction at equilibrium. 2co2 (g) 2co (g) + o2 (g) h° = -514 kj le châtelier's principle predicts that the equilibrium partial pressure of co (g) can be maximized by carrying out the reaction a. at high temperature and high pressure b. at high temperature and low pressure c. at low temperature and low pressure d. at low temperature and high pressure e. in the presence of solid carbon
Answers: 2

Chemistry, 22.06.2019 13:30
Ants live on acacia trees in south america. the ants feed on sugars secreted by the trees. the trees provide room for the ants to live. the ants sting any other insect or animal that comes to eat the trees. what type of relationship is this?
Answers: 1

Chemistry, 22.06.2019 14:50
Given the following information: mass of proton = 1.00728 amu mass of neutron = 1.00866 amu mass of electron = 5.486 × 10^-4 amu speed of light = 2.9979 × 10^8 m/s calculate the nuclear binding energy (absolute value) of 3li^6. which has an atomic mass of 6.015126 amu. j/mol.
Answers: 2
You know the right answer?
Sodium acetate spontaneously crystallizes out of a supersaturated solution upon standing or upon the...
Questions

Business, 21.01.2021 23:00





Mathematics, 21.01.2021 23:00


Mathematics, 21.01.2021 23:00

Mathematics, 21.01.2021 23:00



Biology, 21.01.2021 23:00




Mathematics, 21.01.2021 23:00




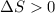 ).
).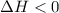 ).
).

