
Cerium (IV) ions are strong oxidizing agents in acid ic solution, oxidizing arsenio us acid to arsen ic acid accor ding to the following equa tion:
2Ce4+(aq)+H3AsO3(aq)+3H2O(l)→2Ce3+( aq)+H3AsO4(aq)+2H+(aq)
A sample of As2O3 weighing 0.217 g is dissolved in basic solution and then acidified to make H3AsO3. Its titration with a solution of acidic cerium{IV) sulfate requires 21.47 ml. Determine the original concentration of Ce^4+(aq) in the titrating solution

Answers: 2
Another question on Chemistry

Chemistry, 21.06.2019 22:30
How much heat is released upon converting one mole of steam (18.0 g) from 100.0 ∘c to water at 25.0 ∘c? show work and constants, trying to figure out how it works. only given the heat capacity for steam and water so try to only use that
Answers: 1

Chemistry, 22.06.2019 04:00
Electric charge is what ? a. kinetic energy b. radiation c. discovery d. electricity
Answers: 1

Chemistry, 22.06.2019 16:40
Identify the lewis acid in this balanced equation: ag+ + 2nh3 -> ag(nh3)2+a. ag+b. nh3c. ag(nh3)2+
Answers: 1

Chemistry, 22.06.2019 18:00
Which three statements represent the benefits of performing experiments using computer simulations?
Answers: 3
You know the right answer?
Cerium (IV) ions are strong oxidizing agents in acid ic solution, oxidizing arsenio us acid to arsen...
Questions

English, 17.06.2020 23:57

English, 17.06.2020 23:57


Mathematics, 17.06.2020 23:57

History, 17.06.2020 23:57


Social Studies, 17.06.2020 23:57



Mathematics, 17.06.2020 23:57



Mathematics, 17.06.2020 23:57


Geography, 17.06.2020 23:57

Mathematics, 17.06.2020 23:57

Mathematics, 17.06.2020 23:57


Mathematics, 17.06.2020 23:57

Mathematics, 17.06.2020 23:57

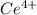 (aq) in the titrating solution.
(aq) in the titrating solution.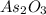 = 0.217 g
= 0.217 g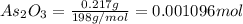
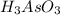 have 1 mole of As.
have 1 mole of As.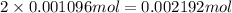 of
of 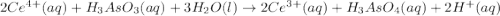
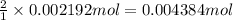 of cerium (IV) ions.
of cerium (IV) ions.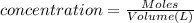
![[Ce^{4+}]=\frac{0.004384 mol}{0.02147 L}=0.2042 M](/tpl/images/0559/2089/fb855.png)


