
A reaction A ( aq ) + B ( aq ) − ⇀ ↽ − C ( aq ) has a standard free‑energy change of − 5.24 kJ / mol at 25 °C. What are the concentrations of A , B , and C at equilibrium if, at the beginning of the reaction, their concentrations are 0.30 M, 0.40 M, and 0 M, respectively?

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 15:20
Identify arrows pointing to bonding electrons. done h-0-0-h ) intro
Answers: 3

Chemistry, 22.06.2019 19:00
Structure of the atoms: discovery of the nucleus in 1909i need answering all of these questions
Answers: 3

Chemistry, 22.06.2019 21:00
Rays from the sun are not considered matter true or false
Answers: 2

Chemistry, 23.06.2019 06:10
2. what two items do autotrophs take from the environment to produce their food? 3. what are the two items that are released during transpiration from leaves? 4. what are the two membranes of the system? a.what are the two stages of photosynthesis? what are the two parts of photosynthesis?
Answers: 2
You know the right answer?
A reaction A ( aq ) + B ( aq ) − ⇀ ↽ − C ( aq ) has a standard free‑energy change of − 5.24 kJ / mol...
Questions




Advanced Placement (AP), 10.09.2019 03:30

Mathematics, 10.09.2019 03:30



Mathematics, 10.09.2019 03:30

Chemistry, 10.09.2019 03:30


Advanced Placement (AP), 10.09.2019 03:30

Mathematics, 10.09.2019 03:30






Computers and Technology, 10.09.2019 03:30


![[A]_{eq}=0.11M](/tpl/images/0559/0215/f777c.png)
![[B]_{eq}=0.21M](/tpl/images/0559/0215/4b396.png)
![[C]_{eq}=0.19M](/tpl/images/0559/0215/f0920.png)
![Kc=exp(-\frac{\Delta _RG }{RT} )=exp[-\frac{-5240J/mol }{(8.314J/mol*K)(298.15K)} ]=8.28](/tpl/images/0559/0215/70087.png)
 due to the chemical reaction, we obtain:
due to the chemical reaction, we obtain: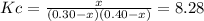
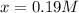
![[A]_{eq}=0.3M-0.19M=0.11M](/tpl/images/0559/0215/f33fb.png)
![[B]_{eq}=0.4M-0.19M=0.21M](/tpl/images/0559/0215/a81c9.png)


