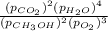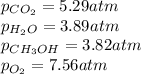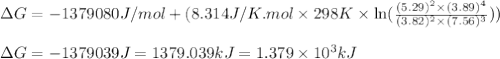A chemist fills a reaction vessel with 3.82 atm methanol (CH, OH) gas, 7.56 am oxygen (O2) gas, 5.29 atm carbon dioxide (CO2) gas, and 3.89 atm water (H0) gas at a temperature of 25.0°C. Under these conditions, calculate the reaction free energy AG for the following chemical reaction: 2CH, OH() + 30266) 2002) + 4H20) Use the thermodynamic information in the ALEKS Data tab. Round your answer to the nearest kilojoule. x 5 ?

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 16:30
Explain in detail of the four major scientific developments that spurred the formulation of the plate tectonics theory
Answers: 2

Chemistry, 22.06.2019 20:00
What happens to the temperature of a substance when the average kinetic energy of its particles increases?
Answers: 3

Chemistry, 22.06.2019 20:30
From the choices provided below, list the reagent(s) in order that will react with cyclopentanone to form the compound shown below.
Answers: 2

Chemistry, 22.06.2019 23:00
Consider the reaction: 2al(s) + fe2o3(s) → al2o3(s) + 2fe(s) the δhf for fe2o3(s) = -824.3 kj/mole. the δhf for al2o3(s) = -1675.7 kj/mole. finish the equation. δhrxn = [(1)( kj/mole) + (2)( kj/mole)] - [(1)( kj/mole) + (2) ( kj/mole)]
Answers: 1
You know the right answer?
A chemist fills a reaction vessel with 3.82 atm methanol (CH, OH) gas, 7.56 am oxygen (O2) gas, 5.29...
Questions






Mathematics, 18.02.2020 20:55



Physics, 18.02.2020 20:55


Mathematics, 18.02.2020 20:55

Computers and Technology, 18.02.2020 20:55





Computers and Technology, 18.02.2020 20:55

Mathematics, 18.02.2020 20:55

Mathematics, 18.02.2020 20:55

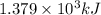
![\Delta G^o_{rxn}=\sum [n\times \Delta G^o_f_{(product)}]-\sum [n\times \Delta G^o_f_{(reactant)}]](/tpl/images/0559/0196/f2395.png)
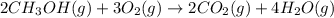
![\Delta G^o_{rxn}=[(2\times \Delta G^o_f_{(CO_2(g))})+(4\times \Delta G^o_f_{(H_2O(g))})]-[(2\times \Delta G^o_f_{(CH_3OH(g))})+(3\times \Delta G^o_f_{(O_2(g))})]](/tpl/images/0559/0196/b933d.png)
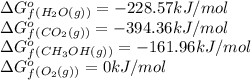
![\Delta G^o_{rxn}=[(2\times (-394.36))+(4\times (-228.57))]-[(2\times (-161.96))+(3\times (0))]\\\\\Delta G^o_{rxn}=-1379.08kJ/mol](/tpl/images/0559/0196/bd425.png)
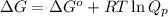
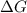 = free energy of the reaction
= free energy of the reaction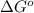 = standard Gibbs free energy = -1379.08 kJ/mol = -1379080 J/mol (Conversion factor: 1 kJ = 1000 J)
= standard Gibbs free energy = -1379.08 kJ/mol = -1379080 J/mol (Conversion factor: 1 kJ = 1000 J)![25^oC=[273+25]K=298K](/tpl/images/0559/0196/0e82f.png)
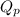 = Ratio of concentration of products and reactants =
= Ratio of concentration of products and reactants =