
Chemistry, 20.03.2020 11:14 ciarrap552
If you assume this reaction is driven to completion because of the large excess of one ion, what is the concentration of [Fe(SCN)]2+ that would be formed from 6.00 mL of 0.00180 M KSCN 5.00 mL 0.240 M Fe(NO3)3 and 14.00 mL of 0.050 M HNO3.
Question 3 options:
0.240 M
4.32 x 10^-4
0.0480
0.0460 M

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 13:50
What happens when an atom of sulfur combines with two atoms of chlorine to produce sci2? a. each chlorine atom shares a pair of electrons with the sulfur atom. b. an electron is transferred from each chlorine atom to the sulfur atom. c. an electron is transferred from the sulfur atom to each chlorine atom. d. each chlorine atom shares all its valence electrons with the sulfur atom.
Answers: 2

Chemistry, 23.06.2019 09:00
Astream of surface water reaches a porous portion of sediment and seeps into the ground. this water eventually joins a large reservoir of water located beneath the earth's surface. the example above describes the interacting with the a. cryosphere; biosphere b. hydrosphere; biosphere c. hydrosphere; geosphere d. cryosphere; geosphere
Answers: 3


Chemistry, 23.06.2019 11:30
Brainliesttt me asap! the below reaction can be categorized as more than one type of reaction. which reactions are these, and what are the types of reactions?
Answers: 1
You know the right answer?
If you assume this reaction is driven to completion because of the large excess of one ion, what is...
Questions






English, 11.09.2019 17:30


Biology, 11.09.2019 17:30




History, 11.09.2019 17:30




History, 11.09.2019 17:30




![[Fe(SCN)]^{2+}](/tpl/images/0556/0307/0c409.png) is,
is, 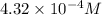
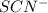 and
and 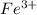 is excess reagent.
is excess reagent.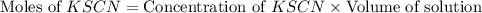
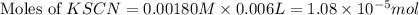
 = Moles of
= Moles of 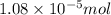
![\text{Concentration of }[Fe(SCN)]^{2+}=\frac{\text{Moles of }[Fe(SCN)]^{2+}}{\text{Volume of solution}}](/tpl/images/0556/0307/e16b4.png)
![\text{Concentration of }[Fe(SCN)]^{2+}=\frac{1.08\times 10^{-5}mol}{0.025L}=4.32\times 10^{-4}M](/tpl/images/0556/0307/cd669.png)


