
Chemistry, 20.03.2020 10:17 ddaaaeeee3503
Romine vapor in a 5.00-liter container at 300 K and 0.205 bar is to be heated to 340 K. Calculate the heat (kJ) that must be transferred to the gas to achieve the desired temperature increase, assuming that ˆ U Uˆ is independent of pressure.

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 11:00
Ais a mountain created from eruptions of lava, ash, rocks, and hot gases.
Answers: 1

Chemistry, 22.06.2019 19:10
Which statement correctly describes the phosphate ion, ? it is composed of one phosphorus atom and four oxygen atoms covalently bonded together, and there is a –3 charge distributed over the entire ion. it is composed of one phosphorus atom and four oxygen atoms covalently bonded together, and there is a –3 charge on the phosphorus atom. it is composed of one phosphorus atom and four oxygen atoms ionically bonded together, and there is a –3 charge distributed over the entire ion. it is composed of one phosphorus atom and four oxygen atoms ionically bonded together, and there is a –3 charge on the phosphorus atom.
Answers: 3

Chemistry, 23.06.2019 03:50
Show how to convert the temperature 84.7° c to kelvin. include all steps and label the final answer.
Answers: 1

You know the right answer?
Romine vapor in a 5.00-liter container at 300 K and 0.205 bar is to be heated to 340 K. Calculate th...
Questions

Spanish, 07.07.2019 03:00



Social Studies, 07.07.2019 03:00

Mathematics, 07.07.2019 03:00

Social Studies, 07.07.2019 03:00

History, 07.07.2019 03:00

Biology, 07.07.2019 03:00


Biology, 07.07.2019 03:00


Social Studies, 07.07.2019 03:00


Advanced Placement (AP), 07.07.2019 03:00

Chemistry, 07.07.2019 03:00



Mathematics, 07.07.2019 03:00


History, 07.07.2019 03:00

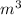
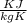
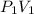 = m R
= m R 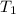
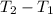 )
)

