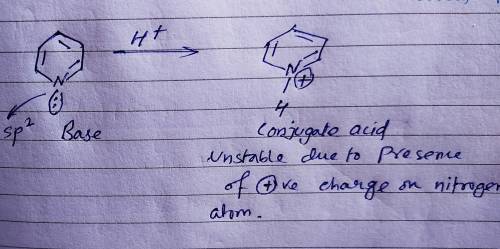
Chemistry, 20.03.2020 09:52 anggelkevin100
Which of the following bases is the WEAKEST? The base is followed by its Kb value. Which of the following bases is the WEAKEST? The base is followed by its Kb value. (CH3CH2)3N, 5.2 × 10-4 C5H5N, 1.7 × 10-9 NH3, 1.76 × 10-5 HOCH2CH2NH2, 3.2 × 10-5 Since these are all weak bases, they have the same strength.

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 12:10
Consider the reaction: n2(g) + o2(g) ⇄ 2no(g) kc = 0.10 at 2000oc starting with initial concentrations of 0.040 mol/l of n2 and 0.040 mol/l of o2, calculate the equilibrium concentration of no in mol/l how would this be done?
Answers: 3

Chemistry, 22.06.2019 12:30
A50.0 ml sample of gas at 20.0 atm of pressure is compressed to 40.0 atm of pressure at constant temperature. what is the new volume? 0.0100 ml 0.325 ml 25.0 ml 100. ml
Answers: 1

Chemistry, 22.06.2019 19:00
Nan element’s square on the periodic table, the number with the greatest numerical value represents the
Answers: 3

Chemistry, 22.06.2019 19:30
Draw the lewis structure for the trisulfur s3 molecule. be sure to include all resonance structures that satisfy the octet rule.
Answers: 3
You know the right answer?
Which of the following bases is the WEAKEST? The base is followed by its Kb value. Which of the foll...
Questions

Mathematics, 20.10.2019 16:30





Chemistry, 20.10.2019 16:30

Mathematics, 20.10.2019 16:30


Mathematics, 20.10.2019 16:30


Mathematics, 20.10.2019 16:30



Mathematics, 20.10.2019 16:30



Mathematics, 20.10.2019 16:30

Spanish, 20.10.2019 16:30


Mathematics, 20.10.2019 16:30