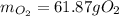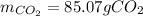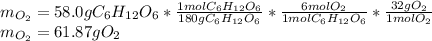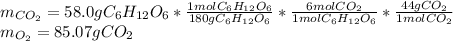Glucose, C 6 H 12 O 6 , is used as an energy source by the human body. The overall reaction in the body is described by the equation C 6 H 12 O 6 ( aq ) + 6 O 2 ( g ) ⟶ 6 CO 2 ( g ) + 6 H 2 O ( l ) Calculate the number of grams of oxygen required to convert 58.0 g of glucose to CO 2 and H 2 O . mass of O 2 : 61.76 g Calculate the number of grams of CO 2 produced.

Answers: 2
Another question on Chemistry

Chemistry, 21.06.2019 13:00
Covalent bonds are formed between metals and boiling points true or false
Answers: 2

Chemistry, 22.06.2019 00:00
How many liters of water vapor can be produced if 108 grams of methane gas (ch4) are combusted at 312 k and 0.98 atm? show all work. pls ! will mark as brainliest
Answers: 1

Chemistry, 22.06.2019 15:30
Each of the following reactions is allowed to come to equilibrium and then the volume is changed as indicated. predict the effect (shift right, shift left, or no effect) of the indicated volume change. drag the appropriate items to their respective bins.co(g) + h2o(g) < => co2(g) + h2(g) (volume is decreased) pcl3(g) + cl2(g) < => pcl5(g) (volume is increased) caco3(s)< => cao(s) + co2(g) (volume is increased)
Answers: 1

Chemistry, 22.06.2019 15:30
The identities of substances are the same before and after which type of change
Answers: 1
You know the right answer?
Glucose, C 6 H 12 O 6 , is used as an energy source by the human body. The overall reaction in the b...
Questions