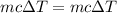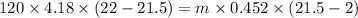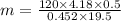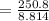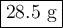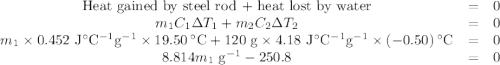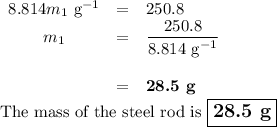Chemistry, 20.03.2020 05:58 soonerlady19
A volume of 120 mL of H2O is initially at room temperature (22.00 ∘C ). A chilled steel rod at 2.00 ∘C ∘C is placed in the water. If the final temperature of the system is 21.50 ∘C ∘C , what is the mass of the steel bar? Use the following values: specific heat of water = 4.18 J/(g⋅∘C)J/(g⋅∘C) specific heat of steel = 0.452 J/(g⋅∘C)

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 12:10
Building glycogen from glucose molecules is an example of
Answers: 3

Chemistry, 22.06.2019 13:30
Which statements are true concerning mineral formation? check all that apply. the slower the cooling, the larger the crystals. the faster the cooling, the smaller the crystals. crystals formed from magma are smaller than crystals formed from lava. minerals can only form in solutions when the solution is heated deep underground. when a solution cools, elements and compounds leave the solution and crystallize as minerals. minerals formed from hot water solutions can form narrow channels in the surrounding rock.
Answers: 1

Chemistry, 22.06.2019 19:30
Chlorine and water react to form hydrogen chloride and oxygen, like this: 2cl2 (g) + 2h2o (g) → 4hcl (g) + o2 (g) also, a chemist finds that at a certain temperature the equilibrium mixture of chlorine, water, hydrogen chloride, and oxygen has the following composition: compound concentration at equilibrium cl2 0.55m h2o 0.57m hcl 0.53m o2 0.34m calculate the value of the equilibrium constant kc for this reaction. round your answer to 2 significant digits.
Answers: 2

Chemistry, 22.06.2019 20:00
For the reaction c6h14(g) & longrightarrow; c6h6(g) + 4h2(g), δp(h2)/δt was found to be 2.5 x 10-2 atm/s, where δp(h2) is the change in pressure of hydrogen. determine δp(c6h14)/δt for this reaction at the same time.
Answers: 2
You know the right answer?
A volume of 120 mL of H2O is initially at room temperature (22.00 ∘C ). A chilled steel rod at 2.00...
Questions


Mathematics, 17.04.2020 20:55



English, 17.04.2020 20:56

Mathematics, 17.04.2020 20:56

Advanced Placement (AP), 17.04.2020 20:56

History, 17.04.2020 20:56


History, 17.04.2020 20:56


Mathematics, 17.04.2020 20:56

Mathematics, 17.04.2020 20:56




Computers and Technology, 17.04.2020 20:56



Mathematics, 17.04.2020 20:56