Chemistry, 20.03.2020 05:50 jonystroyer1020
Given that a chlorine-oxygen bond has an enthalpy of 243 kJ/mol , an oxygen-oxygen bond has an enthalpy of 498 kJ/mol , and the standard enthalpy of formation of ClO2 102.5 kJ/mol , calculate the value for the enthalpy of formation per mole of ClO(g). What is the value for the enthalpy of formation per mole of ClO(g)?

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 06:30
This drawing shows a human body system. what is the primary function of this body system?
Answers: 3

Chemistry, 22.06.2019 08:00
If 90.0 grams of ethane reacted with excess chlorine,how many grams of dicarbon hexachloride would form
Answers: 1

Chemistry, 22.06.2019 09:30
One way that radioactive waste is treated is by burying it in repositories. the repositories are found only in states with very low populations. true or false? a. trueb. false(also i meant to put high school but it put down middle school instead)
Answers: 1

Chemistry, 22.06.2019 10:30
If you add 5.00 ml of 0.100 m sodium hydroxide to 50.0 ml of acetate buffer that is 0.100 m in both acetic acid and sodium acetate, what is the ph of the resulting solution? acetic acid: ka = 1.8. x 10-5
Answers: 1
You know the right answer?
Given that a chlorine-oxygen bond has an enthalpy of 243 kJ/mol , an oxygen-oxygen bond has an entha...
Questions


Mathematics, 06.11.2019 17:31


English, 06.11.2019 17:31

Mathematics, 06.11.2019 17:31

Mathematics, 06.11.2019 17:31

Mathematics, 06.11.2019 17:31


Mathematics, 06.11.2019 17:31

Mathematics, 06.11.2019 17:31

Social Studies, 06.11.2019 17:31









Chemistry, 06.11.2019 17:31

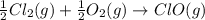
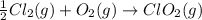 ;
; 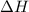 = 102.5 kJ
= 102.5 kJ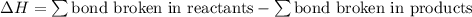
![[(\frac{1}{2})x + 498] - [(2)(243)]](/tpl/images/0555/5141/c814b.png)
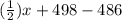
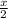
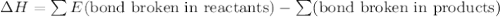
![[(\frac{1}{2})181 + (\frac{1}{2})498] - 243](/tpl/images/0555/5141/97422.png)