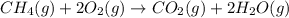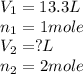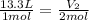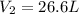Answers: 2
Another question on Chemistry

Chemistry, 21.06.2019 14:00
What is the molar mass of babr2? a. 217.2 g/mol b. 297.1 g/mol c. 354.5 g/mol d. 434.4 g/mol
Answers: 1

Chemistry, 22.06.2019 06:30
Predict whether the changes in enthalpy, entropy, and free energy will be positive or negative for the boiling of water, and explain your predictions. how does temperature affect the spontaneity of this process?
Answers: 1

Chemistry, 22.06.2019 13:30
Ants live on acacia trees in south america. the ants feed on sugars secreted by the trees. the trees provide room for the ants to live. the ants sting any other insect or animal that comes to eat the trees. what type of relationship is this?
Answers: 1

Chemistry, 22.06.2019 13:50
How does the motion of particles in a gas change as the gas cools
Answers: 2
You know the right answer?
How many liters of water vapor can be produced if 13.3 liters of methane gas (ch4) are combusted, if...
Questions

Biology, 05.07.2019 07:30



English, 05.07.2019 07:30

Mathematics, 05.07.2019 07:30

Mathematics, 05.07.2019 07:30

English, 05.07.2019 07:30

Mathematics, 05.07.2019 07:30


Mathematics, 05.07.2019 07:30

Mathematics, 05.07.2019 07:30





History, 05.07.2019 07:30




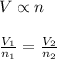
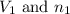 = Initial volume and number of moles
= Initial volume and number of moles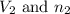 = Final volume and number of moles
= Final volume and number of moles