
Chemistry, 19.03.2020 23:13 esmeralda266
Chloride anions in solution will combine with the silver cations to produce bright white silver chloride precipitate. Suppose an EPA chemist tests a 250.mL sample of groundwater known to be contaminated with iron(III) chloride, which would react with silver nitrate solution like this:FeCl3(aq) + 3AgNO3(aq) ⟶ 3AgCl(s) + FeNO3(aq)The chemist adds 82.0 M silver nitrate solution to the sample until silver chloride stops forming. He then washes, dries, and weighs the precipitate. He finds he has collected 2.5mg of silver chloride. Calculate the concentration of iron(III) chloride contaminant in the original groundwater sample.

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 03:10
Between 2014 and 2016, more than 25,000 children in flint, michigan, drank water that was contaminated with lead from lead pipes. during this time, the city claimed the water was safe to drink. which of these actions could the city have taken to ensure that the drinking water was free from lead?
Answers: 3

Chemistry, 22.06.2019 03:30
Calculate the molar mass of aluminum oxide (al2o3). express your answer to four significant figures.
Answers: 1

Chemistry, 22.06.2019 05:40
Salicylic acid is a very important acid. it is used to synthesize the aspirin by treating with acetic anhydride. a 0.2015-g sample of salicylic acid was dissolved in a 100.00-ml volumetric flask, and the solution was diluted to the mark. a 10-ml aliquot of this solution was titrated with standard naoh (0.01130 + 0.2% n) to a phenolphthalein faint pink color end point at 19.81 ml. (a) (calculate the normality of the salicylic acid solution used in the titration. (b) assuming the salicylic acid is pure, what is the equivalent weight of the salicylic acid? practice problems for the final exam (continued) (c) (calculate the inherent error in the determination of the equivalent weight you calculated in part (b). use the following absolute errors in the equipment /glassware when calculating the inherent error. 5.00-ml pipet: + 0.02 ml 100-ml volumetric flask: + 0.08 ml analytical balance: + 0.2 mg 25-ml buret: + 0.03 ml
Answers: 2

Chemistry, 22.06.2019 18:30
Which sample at stp has the same number of atoms as 18 liters of ne at stp
Answers: 1
You know the right answer?
Chloride anions in solution will combine with the silver cations to produce bright white silver chlo...
Questions

Physics, 08.12.2021 02:50


Mathematics, 08.12.2021 02:50

Mathematics, 08.12.2021 02:50

Mathematics, 08.12.2021 02:50

Mathematics, 08.12.2021 02:50

History, 08.12.2021 02:50


Mathematics, 08.12.2021 02:50

Mathematics, 08.12.2021 02:50





Social Studies, 08.12.2021 02:50





Advanced Placement (AP), 08.12.2021 02:50

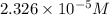 the concentration of iron(III) chloride contaminant in the original groundwater sample.
the concentration of iron(III) chloride contaminant in the original groundwater sample.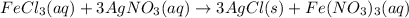
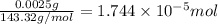
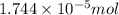 od silver chloride will be obtained from ;
od silver chloride will be obtained from ;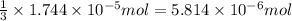 of ferric chloride
of ferric chloride![[FeCl_3]=\frac{5.814\times 10^{-6} mol}{0.250 L}=2.326\times 10^{-5} M](/tpl/images/0554/8189/91194.png)


