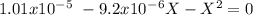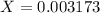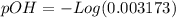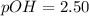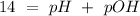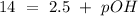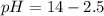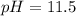Chemistry, 19.03.2020 22:19 nativebabydoll35
The base protonation constant Kb of allantoin (C4H4N3O3NH2) is ×9.1210−6. Calculate the pH of a 1.1M solution of allantoin at 25°C. Round your answer to 1 decimal pl

Answers: 3
Another question on Chemistry

Chemistry, 21.06.2019 14:10
Amonoprotic acid is an acid that donates a single proton to the solution. suppose you have 0.140 g of a monoprotic acid dissolved in 35.0 ml of water. this solution is then neutralized with 14.5 ml of 0.110 m naoh. what is the molar mass of the acid?
Answers: 1

Chemistry, 22.06.2019 06:00
Calculate - analysis of compound composed of iron and oxygen yields 174.86 of fe and 75.14g of o. what is the empirical formula for this compound?
Answers: 3

Chemistry, 22.06.2019 18:30
The table lists the lattice energies of some compounds.compoundlattice energy (kj/mol)lif –1,036licl –853naf –923kf –821nacl –786which statement about crystal lattice energy is best supported by the information in the table? the lattice energy increases as cations get smaller, as shown by lif and kf.the lattice energy increases as the cations get larger, as shown by lif and licl.the lattice energy decreases as cations get smaller, as shown by nacl and naf.the lattice energy decreases as the cations get smaller, as shown by naf and kf.
Answers: 3

Chemistry, 22.06.2019 21:30
How can the periodic table be used to predict the behavior of elements?
Answers: 1
You know the right answer?
The base protonation constant Kb of allantoin (C4H4N3O3NH2) is ×9.1210−6. Calculate the pH of a 1.1M...
Questions

English, 20.12.2020 04:40





Health, 20.12.2020 04:50

Social Studies, 20.12.2020 04:50

Mathematics, 20.12.2020 04:50

Mathematics, 20.12.2020 04:50



Law, 20.12.2020 04:50


Mathematics, 20.12.2020 04:50

Mathematics, 20.12.2020 04:50



Mathematics, 20.12.2020 04:50

Mathematics, 20.12.2020 04:50

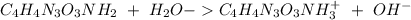
![Kb=\frac{[C_4H_4N_2O_3NH_3^+][OH^-]}{[C_4H_4N_3O_3NH_2]}](/tpl/images/0554/7437/19295.png)
![9.2x10^-^6=\frac{[X][X]}{[1.1-X]}](/tpl/images/0554/7437/d576a.png)
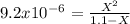
![(9.2x10^-^6)*[1.1-X]=X^2](/tpl/images/0554/7437/e621d.png)