Consider this reaction:
2H3PO4(aq)→ P2O5(aq)+ 3H2O
At a certain temperature it ob...

Chemistry, 19.03.2020 17:16 jadielmatmat
Consider this reaction:
2H3PO4(aq)→ P2O5(aq)+ 3H2O
At a certain temperature it obeys this rate law. rate
rate= (46.6M^-1. s^-1) [H3PO4]^2
Suppose a vessel contains H3PO4 at a concentration of 0.660M. Calculate how long it takes for the concentration of H3PO$ to decrease to 20% to its natural value. You may assume no other reaction is important. Round your answer to significant digits.

Answers: 3
Another question on Chemistry

Chemistry, 21.06.2019 18:00
Write a net ionic equation to show that hydrofluoric acid, behaves as an acid in water.
Answers: 1

Chemistry, 22.06.2019 02:20
6. what does the symbol ah stand for? o one calorie given off by a reaction the specific heat of a substance the heat capacity of a substance the heat of reaction for a chemical reaction
Answers: 1

Chemistry, 22.06.2019 12:30
The melting point of sulfur is 115 °c and its boiling point is 445 °c. what state would sulfur be in at 200 °c?
Answers: 1

Chemistry, 22.06.2019 13:00
One of the hopes for solving the world's energy problem is to make use of the fusion reaction 21h +31h --> 42he + 10n + energy how much energy is released when 1 mol of deuterium is fused with 1 mol of tritium according to the above reaction? the masses of the atoms and the neutrons are as follows: 21h = 2.0140 amu 31h = 3.01605 amu 42he = 4.002603 amu 10n = 1.008665 amu. the speed of light is 2.9979 x 108 m/s.
Answers: 1
You know the right answer?
Questions

Mathematics, 03.12.2019 17:31

Computers and Technology, 03.12.2019 17:31

History, 03.12.2019 17:31


Mathematics, 03.12.2019 17:31



History, 03.12.2019 17:31

Chemistry, 03.12.2019 17:31


Mathematics, 03.12.2019 17:31

English, 03.12.2019 17:31


History, 03.12.2019 17:31

Mathematics, 03.12.2019 17:31




Mathematics, 03.12.2019 17:31

![Rate=k[H_3PO_4]^2](/tpl/images/0554/2402/79104.png)
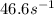
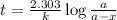
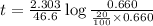
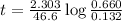
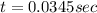
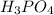 to decrease to 20% to its natural value is 0.0345 sec
to decrease to 20% to its natural value is 0.0345 sec

