
Chemistry, 19.03.2020 08:58 jetblackcap
Phosphorus pentachloride decomposes according to the chemical equation PCl 5 ( g ) − ⇀ ↽ − PCl 3 ( g ) + Cl 2 ( g ) K c = 1.80 at 250 ∘ C A 0.1846 mol sample of PCl 5 ( g ) is injected into an empty 2.55 L reaction vessel held at 250 ∘ C. Calculate the concentrations of PCl 5 ( g ) and PCl 3 ( g ) at equilibrium.

Answers: 2
Another question on Chemistry

Chemistry, 21.06.2019 23:00
Write a brief passage describing a neutral atom of nitrogen-14 (n-14). describe the number of protons, neutrons, and electrons in the atom, where each type of particle is located, and how the terms atomic number, mass number, and atomic mass are related to the particles. use the periodic table to you. 14 protons and eletrons since its a neutral atom
Answers: 1

Chemistry, 22.06.2019 09:30
1. explain hydrogen peroxide, h 2 o 2 properties and decomposition reaction. 2. describe how each of the following natural cycles plays a part in earth’s climate system. (a) the water cycle (b) the carbon cycle
Answers: 1

Chemistry, 23.06.2019 08:00
Technician a says that you should never jump-start a frozen battery. technician b says that a frozen battery can explode, causing injury, when jump-started. who is correct?
Answers: 2

Chemistry, 23.06.2019 11:30
Which part of the healthcare system could best explain how a pharmaceutical drug works
Answers: 2
You know the right answer?
Phosphorus pentachloride decomposes according to the chemical equation PCl 5 ( g ) − ⇀ ↽ − PCl 3 ( g...
Questions


Mathematics, 29.01.2021 21:50

Mathematics, 29.01.2021 21:50


Mathematics, 29.01.2021 21:50



History, 29.01.2021 21:50

Geography, 29.01.2021 21:50

Physics, 29.01.2021 21:50


Mathematics, 29.01.2021 21:50





Mathematics, 29.01.2021 21:50


Social Studies, 29.01.2021 21:50

Mathematics, 29.01.2021 21:50

![[PCl_3]_{eq}=0.0697M\\](/tpl/images/0553/8714/e4a7f.png)
![[PCl_5]_{eq}=0.00269M](/tpl/images/0553/8714/6813e.png)
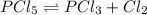
![[PCl_5]_0=\frac{0.1846mol}{2.55L}=0.0724M](/tpl/images/0553/8714/c9006.png)
![Kc=\frac{[Cl_2]_{eq}[PCl_3]_{eq}}{[PCl_5]_{eq}}](/tpl/images/0553/8714/38534.png)
 due to the reaction extent, it becomes:
due to the reaction extent, it becomes: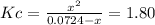
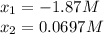
![[PCl_3]_{eq}=x=0.0697M\\](/tpl/images/0553/8714/b7f0d.png)
![[PCl_5]_{eq}=0.0724M-x=0.0724M-0.0697M=0.00269M](/tpl/images/0553/8714/fc102.png)


