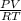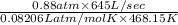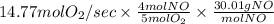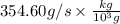Chemistry, 19.03.2020 08:01 leomessifanboy678
Suppose a chemical engineer studying a new catalyst for the oxidation of ammonia reaction finds that liters per second of dioxygen are consumed when the reaction is run at and . Calculate the rate at which nitrogen monoxide is being produced. Give your answer in kilograms per second. Round your answer to significant digits.

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 00:40
Which is a difference between molecular compounds and ionic compounds? select the correct answer below: question 5 options: molecular compounds typically form between a metal and a nonmetal, while ionic compounds typically form between nonmetals. molecular compounds result from the transfer of electrons between atoms to form ions, while ionic compounds result from the sharing of electrons between neutral atoms. molecular compounds are formed of discrete, neutral molecules, while ionic compounds are formed of large repeating arrays of opposite charges. molecular compounds have high melting points and high boiling points, while ionic
Answers: 3

Chemistry, 22.06.2019 22:30
Which of the following is true about the speed of light? it depends on the wavelength.
Answers: 3

Chemistry, 23.06.2019 02:00
Which statement is true about the model of the electromagnetic spectrum a: it change the frequencies of light. b: it compare wavelengths of light. c: the color of light waves can be changed using the model. d: the intensities of light waves can be decreased using the model.
Answers: 2

Chemistry, 23.06.2019 04:00
If you are told to get 100 ml of stock solution to use to prepare smaller size sample for an experiment, which piece of glassware would you use?
Answers: 1
You know the right answer?
Suppose a chemical engineer studying a new catalyst for the oxidation of ammonia reaction finds that...
Questions

Mathematics, 27.06.2020 15:01




Mathematics, 27.06.2020 15:01











World Languages, 27.06.2020 15:01



English, 27.06.2020 15:01

English, 27.06.2020 15:01

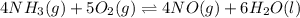
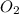 per second consumed is as follows.
per second consumed is as follows.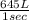
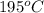 (468.15 K) and pressure 0.88 atm. Hence, moles of consumption of
(468.15 K) and pressure 0.88 atm. Hence, moles of consumption of