
A piece of iron (mass = 25.0 g) at 398 K is placed in a styrofoam coffee cup containing 25.0 mL of water at 298 K. Assuming that no heat is lost to the cup or the surroundings, what will the final temperature of the water be? The specific heat capacity of iron = 0.449 J/g°C and water = 4.18 J/g°C.

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 06:00
An atom of sodium-23 (atomic number = 11) has a positive charge of +1. give this information, how many electrons does it have? how many proteins and neutrons does this atom have
Answers: 2

Chemistry, 22.06.2019 20:00
How are the terms group and period used on the periodic table
Answers: 1

Chemistry, 23.06.2019 00:30
Gasoline has a density of 0.740 g/ml. if you have 328 grams of gasoline, what is the volume in milliliters?
Answers: 1

Chemistry, 23.06.2019 01:00
Which description best characterization the motion of particles in a solid
Answers: 1
You know the right answer?
A piece of iron (mass = 25.0 g) at 398 K is placed in a styrofoam coffee cup containing 25.0 mL of w...
Questions


Computers and Technology, 25.03.2020 22:29


English, 25.03.2020 22:29



History, 25.03.2020 22:29


English, 25.03.2020 22:29

Mathematics, 25.03.2020 22:29


Mathematics, 25.03.2020 22:29

Mathematics, 25.03.2020 22:29

Mathematics, 25.03.2020 22:29




Mathematics, 25.03.2020 22:29

Mathematics, 25.03.2020 22:29

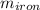 = 25 gm
= 25 gm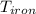 = 398 K
= 398 K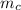 = 25 ml = 25 gm
= 25 ml = 25 gm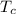 = 298 K
= 298 K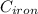 = 0.449
= 0.449 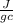
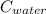 = 4.18
= 4.18 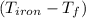 =
= 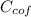 ×
× 
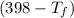 = 25 × 4.18 ×
= 25 × 4.18 × 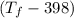
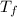 = 9.3 (
= 9.3 ( 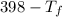 = 9.3
= 9.3 

