
When 0.243 g of Mg metal is combined with enough HCl to make 100 mL of solution in a constant-pressure calorimeter, the following reaction occurs: Mg(s) + 2 HCl(aq) → MgCl2(aq) + H2(g) If the temperature of the solution increases from 23.0 ∘C to 34.1 ∘C as a result of this reaction, calculate ΔH in kJ/mol of Mg. Assume that the solution has a specific heat of 4.18 J/g∘C.

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 02:30
Margaret wants to make an orange flavored drink by stirring powdered drink mix into a glass of water. she doesn't like drinks that have small clumps of powdered solid in them, so she wants the drink to be a perfect solution. what factors should margaret not consider when deciding how much powder to add to her glass of water?
Answers: 3

Chemistry, 22.06.2019 18:30
When the chemicals iron sulfide (fes) and hydrochloric acid (hcl) are combined, bubbles appear from the mixture. 1. does the appearance of bubbles indicate a physical or chemical change? 2. why do the bubbles indicate this change? 3. what property is this?
Answers: 1

Chemistry, 22.06.2019 22:10
Determine the ph of 0.10 m nh3 solution. nh3 is a weak base with a kb equal to 1.8 x 10-5 round answer to nearest whole number.
Answers: 1

Chemistry, 23.06.2019 02:00
The plant food contains nh4)3po4 what tests would you run to verify the presence of the nh4 ion and the po4 ion
Answers: 2
You know the right answer?
When 0.243 g of Mg metal is combined with enough HCl to make 100 mL of solution in a constant-pressu...
Questions

Mathematics, 08.01.2021 21:00


Mathematics, 08.01.2021 21:00



Mathematics, 08.01.2021 21:00


Biology, 08.01.2021 21:00

Mathematics, 08.01.2021 21:00

Geography, 08.01.2021 21:00

Engineering, 08.01.2021 21:00

Mathematics, 08.01.2021 21:00


Mathematics, 08.01.2021 21:00


Mathematics, 08.01.2021 21:00

Mathematics, 08.01.2021 21:00



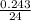 = 0.01 mol
= 0.01 mol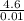 = 460 kj mol⁻¹
= 460 kj mol⁻¹

