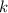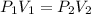A certain amount of chlorine gas was placed inside a cylinder with a movable piston at one end. The initial volume was 3.00 L and the initial pressure of chlorine was 1.85 atm . The piston was pushed down to change the volume to 1.00 L. Calculate the final pressure of the gas if the temperature and number of moles of chlorine remain constant

Answers: 2
Another question on Chemistry

Chemistry, 21.06.2019 15:30
1. baking powder is a 1: 1 molar mixture of cream of tartar (khc4h4o6) and baking soda (nahco3). a recipe calls for two teaspoons (a total of 8.0 grams) of cream of tartar. how much baking soda must be added for both materials to react completely?
Answers: 2


Chemistry, 22.06.2019 00:30
This element exists in adundance in the sun.explain how you would go about capturing sunlight.would this captured sunlight contain any of the element?
Answers: 1

Chemistry, 22.06.2019 09:20
Explain that newton first law,second law and third law of motion?
Answers: 2
You know the right answer?
A certain amount of chlorine gas was placed inside a cylinder with a movable piston at one end. The...
Questions



Spanish, 21.04.2020 23:25


Social Studies, 21.04.2020 23:25


English, 21.04.2020 23:25


Mathematics, 21.04.2020 23:25


Mathematics, 21.04.2020 23:25




Social Studies, 21.04.2020 23:25

English, 21.04.2020 23:25

Chemistry, 21.04.2020 23:25



English, 21.04.2020 23:25

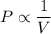
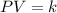 Pressure multiplied by volume equals some constant
Pressure multiplied by volume equals some constant