

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 09:10
Select the correct answer from each drop-down menu.describe what happens to a carbon-11 atom when it undergoes positron emission.the decay of a carbon-11 atom _1_, and this causes it to emit _2_.options for 1: > changes a neutron into a proton> changes a proton into a neutron> is hit with a neutron> reconfigures its protons and neutronsoptions for 2: > a negatively charged electron-sized particle> a positively charged election-sized particle> two atoms and several neutrons> two neutrons and two protons
Answers: 3

Chemistry, 22.06.2019 13:00
12. calculate the hydroxide ion concentration of a solution with ph = 3.25. show all calculations leading to an answer
Answers: 3

Chemistry, 22.06.2019 22:00
4.25g sample of solid ammonium nitrate dissolves in 60.0g of water in a coffee-cup calorimeter, the temperature drops from 22.0 c to 16.9 c. assume that the specific heat of the solution is the same as that of pure water. calculate delta(h) (in kj/mol nh4no3) for the solution proces.
Answers: 2

Chemistry, 23.06.2019 04:20
The graph shows one consequence of urban sprawl. how did urban sprawl contribute to the change in biodiversity
Answers: 2
You know the right answer?
Determine the change in entropy for 2.7 moles of an ideal gas originally placed in a container with...
Questions


Mathematics, 18.03.2021 02:40


Mathematics, 18.03.2021 02:40

Arts, 18.03.2021 02:40

Mathematics, 18.03.2021 02:40







History, 18.03.2021 02:40



Arts, 18.03.2021 02:40

Biology, 18.03.2021 02:40



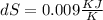
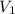 = 4 L
= 4 L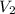 = 6 L
= 6 L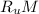
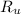 = Universal gas constant = 8.314
= Universal gas constant = 8.314 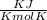
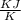
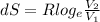
![dS = 0.0224 log _{e} [\frac{6}{4}]](/tpl/images/0550/1275/25cc6.png)


