
Chemistry, 17.03.2020 00:54 kprincess16r
A certain metal M crystallizes in a lattice described by a body-centered cubic (bcc) unit cell. The lattice constant (a, the edge of the unite cell) has been measured by X-ray crystallography to be 360. pm. Calculate the radius of an atom of M. Be sure your answer has 3 significant digits, and be sure it has the correct unit symbol.

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 04:30
How many grams of co(g) are there in 74.5 ml of the gas at 0.933 atm and 30o c?
Answers: 1


Chemistry, 22.06.2019 13:50
Abeaker with 2.00×102 ml of an acetic acid buffer with a ph of 5.000 is sitting on a benchtop. the total molarity of acid and conjugate base in this buffer is 0.100 m. a student adds 4.70 ml of a 0.360 m hcl solution to the beaker. how much will the ph change? the pka of acetic acid is 4.740.
Answers: 1

Chemistry, 22.06.2019 15:00
How is the shape of the poem “peer” connected to its meaning?
Answers: 2
You know the right answer?
A certain metal M crystallizes in a lattice described by a body-centered cubic (bcc) unit cell. The...
Questions


Geography, 29.09.2019 20:50

Health, 29.09.2019 20:50

Physics, 29.09.2019 20:50


Geography, 29.09.2019 20:50

Mathematics, 29.09.2019 20:50

Mathematics, 29.09.2019 20:50

Mathematics, 29.09.2019 20:50



Mathematics, 29.09.2019 20:50


English, 29.09.2019 20:50



Mathematics, 29.09.2019 20:50


Biology, 29.09.2019 20:50

English, 29.09.2019 20:50

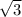 /4
/4

