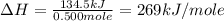Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 09:30
Which ocean zone has the most abundant primary producer and why a) the abyssopelagic zone ,du to the absence of light and cold water temperatureb) the bathypelagic zone, due to the absence of light and cold water temperaturec) the mesopelagic zone ,due to uts high light availability and warm water temperature d) the epipelagic zone,due to its high light availability and warm water temperature
Answers: 3

Chemistry, 22.06.2019 21:20
Phosgene (carbonyl chloride), cocl2, is an extremely toxic gas that is used in manufacturing certain dyes and plastics. phosgene can be produced by reacting carbon monoxide and chlorine gas at high temperatures: co(g) cl2(g)⇌cocl2(g) carbon monoxide and chlorine gas are allowed to react in a sealed vessel at 477 ∘c . at equilibrium, the concentrations were measured and the following results obtained: gas partial pressure (atm) co 0.830 cl2 1.30 cocl2 0.220 what is the equilibrium constant, kp, of this reaction
Answers: 2

Chemistry, 22.06.2019 23:00
What is the most common reason for matter changing its state?
Answers: 1

Chemistry, 23.06.2019 01:30
How is the solubility of a carbon dioxide gas in water increase?
Answers: 1
You know the right answer?
A bomb calorimeter has a heat capacity of 675 J/°C and contains 925 g of water. If the combustion of...
Questions

English, 13.05.2021 01:50

Arts, 13.05.2021 01:50

Mathematics, 13.05.2021 01:50






Physics, 13.05.2021 01:50



Mathematics, 13.05.2021 01:50




Arts, 13.05.2021 01:50

Mathematics, 13.05.2021 01:50


English, 13.05.2021 01:50

Mathematics, 13.05.2021 01:50

![q=[q_1+q_2]](/tpl/images/0549/6528/341bc.png)
![q=[c_1\times \Delta T+m_2\times c_2\times \Delta T]](/tpl/images/0549/6528/1d50b.png)
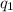 = heat absorbed by the calorimeter
= heat absorbed by the calorimeter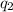 = heat absorbed by the water
= heat absorbed by the water = specific heat of calorimeter =
= specific heat of calorimeter = 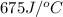
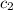 = specific heat of water =
= specific heat of water = 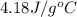
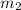 = mass of water = 925 g
= mass of water = 925 g = change in temperature =
= change in temperature = 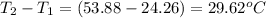
![q=[(675J/^oC\times 29.62^oC)+(925g\times 4.18J/g^oC\times 29.62^oC)]](/tpl/images/0549/6528/f9109.png)
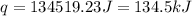
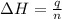
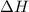 = enthalpy change = ?
= enthalpy change = ?