
Chemistry, 16.03.2020 23:45 alejandramirand9836
A cylinder with a moveable piston contains 92g of Nitrogen. The external pressure is constant at 1.00 atm. The initial temperature is 200K. When the temperature is decreased by 85 K, by putting it in a lower temperature freezer, the volume will decrease, according to the Ideal Gas Law. Calculate the work for this process. Express your answer in J. The conversion factor between liter atmospheres and joules is 101.3 J

Answers: 3
Another question on Chemistry

Chemistry, 21.06.2019 22:30
Ionic compounds are made of ions, and yet the overall charge of an ionic compound is neutral. why?
Answers: 1

Chemistry, 22.06.2019 04:00
The rules of engagement (roe) working group is often used to (select all that apply.)
Answers: 2

Chemistry, 22.06.2019 11:30
If blood contains 150g of hemoglobin per liter of blood, how much hemoglobin would be contained in 10 ml of blood
Answers: 2

Chemistry, 22.06.2019 19:30
Anurse used a 0.02-mg/l solution of disinfection to clean a patients wound. what is the concentration of the solution expressed as a percentage?
Answers: 1
You know the right answer?
A cylinder with a moveable piston contains 92g of Nitrogen. The external pressure is constant at 1.0...
Questions

English, 22.09.2019 13:50

History, 22.09.2019 13:50


English, 22.09.2019 13:50




History, 22.09.2019 13:50


Health, 22.09.2019 13:50



Mathematics, 22.09.2019 13:50



Mathematics, 22.09.2019 13:50

History, 22.09.2019 13:50

Mathematics, 22.09.2019 13:50


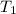 = 200 K
= 200 K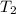 = 200 - 85 = 115 K
= 200 - 85 = 115 K
 =
=  ------------ ( 1 )
------------ ( 1 )
 = m R
= m R 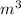
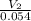 =
= 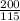
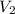 = 0.094
= 0.094 

