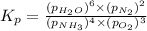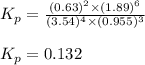Chemistry, 16.03.2020 22:22 fordalolz1553
Problem Page Question Ammonia has been studied as an alternative "clean" fuel for internal combustion engines, since its reaction with oxygen produces only nitrogen and water vapor, and in the liquid form it is easily transported. An industrial chemist studying this reaction fills a flask with of ammonia gas and of oxygen gas, and when the mixture has come to equilibrium measures the partial pressure of water vapor to be . Calculate the pressure equilibrium constant for the combustion of ammonia at the final temperature of the mixture. Round your answer to significant digits. Clears your work. Undoes your last action. Provides information about entering answers.

Answers: 2
Another question on Chemistry


Chemistry, 22.06.2019 14:00
The content of manganese (mn) in steel was determined spectrophotometrically and with the use of the standard addition method. an unknown sample of mn from a digested steel sample gave an absorbance of 0.185 when analyzed spectrophotometrically. when 5.00 ml of solution containing 95.5 ppm mn was added to 50.0 ml of the unknown steel solution (digested sample), the absorbance was 0.248. calculate the concentration, in parts-per-million (ppm), of mn in the digested steel sample solution.
Answers: 3

Chemistry, 22.06.2019 18:30
How many moles of lead are in 1.50 x 10^12 atoms of lead? could you explain the answer as well and not just give it to me i am refreshing for finals and i need to know how to do it
Answers: 3

You know the right answer?
Problem Page Question Ammonia has been studied as an alternative "clean" fuel for internal combustio...
Questions

English, 09.12.2019 03:31



Mathematics, 09.12.2019 03:31

Mathematics, 09.12.2019 03:31




Arts, 09.12.2019 03:31






History, 09.12.2019 03:31




Mathematics, 09.12.2019 03:31

English, 09.12.2019 03:31

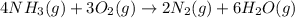
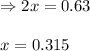
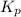 for above equation follows:
for above equation follows: