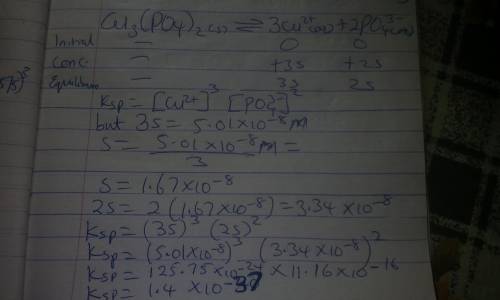You add 10.0 grams of solid copper(II) phosphate, Cu3(PO4)2, to a beaker and then add 100.0 mL of water to the beaker at T = 298 K. The solid does not appear to dissolve. You wait a long time, with occasional stirring and eventually measure the equilibrium concentration of Cu2+(aq) in the water to be 5.01×10−8 M. What is the Ksp of copper(II) phosphate?

Answers: 2
Another question on Chemistry

Chemistry, 21.06.2019 22:40
How many electrons does silver have to give up in order to achieve a sido noble gas electron configuration
Answers: 1

Chemistry, 22.06.2019 08:00
What is the molarity of 60.0 grams of naoh dissolved in 750 milliliters of water? a) 1.1 m b) 2.0 m c) 12 m d) 75 m
Answers: 1

Chemistry, 22.06.2019 13:00
Jose and eric were given four samples in lab. the results of their analysis are shown in the table. based on the data they collected, which sample is most likely a metal?
Answers: 1

Chemistry, 23.06.2019 01:00
Which elements are found in glucose, the product of photosynthesis? a. carbon, hydrogen, and oxygen b. carbon and hydrogen c. carbon, nitrogen, and oxygen d. hydrogen, nitrogen, and carbon
Answers: 2
You know the right answer?
You add 10.0 grams of solid copper(II) phosphate, Cu3(PO4)2, to a beaker and then add 100.0 mL of wa...
Questions

Mathematics, 04.06.2021 23:40


Mathematics, 04.06.2021 23:40

Mathematics, 04.06.2021 23:40

Social Studies, 04.06.2021 23:40

Mathematics, 04.06.2021 23:40





History, 04.06.2021 23:50




Mathematics, 04.06.2021 23:50


Mathematics, 04.06.2021 23:50

Biology, 04.06.2021 23:50


Mathematics, 04.06.2021 23:50