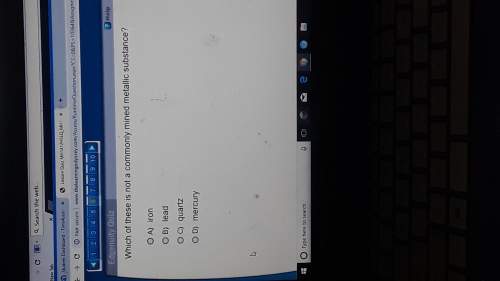
Which of the following statements is false? A) A reaction may not occur at a detectable rate even though it has a favorable equilibrium. B) After a reaction, the enzyme involved becomes available to catalyze the reaction again. C) For S → P, a catalyst shifts the reaction equilibrium to the right. D) Lowering the temperature of a reaction will lower the reaction rate. E) Substrate binds to an enzyme's active site.

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 11:40
Calculate the number of kilojoules to warm 125 g of iron from 23.5°c to 78.0°c.
Answers: 3

Chemistry, 22.06.2019 14:30
How does a noncompetitive inhibitor reduce an enzyme’s activity?
Answers: 1

Chemistry, 23.06.2019 09:30
Large crystals are formed when igneous rocks cool very slowly igneous rocks cool very quickly sedimentary rock is eroded metamorphic rocks change into igneous rock
Answers: 1

Chemistry, 23.06.2019 10:00
Why sncl2 is solid while sncl4 is liquid at room temprature explain it in easy way
Answers: 1
You know the right answer?
Which of the following statements is false? A) A reaction may not occur at a detectable rate even th...
Questions

Spanish, 03.08.2019 21:30

Mathematics, 03.08.2019 21:30


History, 03.08.2019 21:30

Biology, 03.08.2019 21:30


French, 03.08.2019 21:30


English, 03.08.2019 21:30


Mathematics, 03.08.2019 21:30

History, 03.08.2019 21:30








Chemistry, 03.08.2019 21:30