
Chemistry, 16.03.2020 18:30 Fireburntbudder
This reaction is carried out at a different temperature with initial concentrations of [CO]=0.27M and [H2]=0.49M. At equilibrium, the concentration of CH3OH is 0.11 M. Find the equilibrium constant at this temperature.

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 04:30
Use the drop-down menus to answer each question. which runner finished the 100 m race in the least amount of time? which runner stopped running for a few seconds during the race? at what distance did anastasia overtake chloe in the race?
Answers: 1

Chemistry, 22.06.2019 21:00
Use the measurements in the table to determine which unidentified metal has the highest density. metal volume mass a 10.5 cm3 122 g b 14.2 cm3 132 g c 16.1 cm3 115 g d 12.7 cm3 126 g
Answers: 2


Chemistry, 23.06.2019 02:00
Which would freeze at a higher temperature: the great salt lake or lake tahoe? a. lake tahoe would freeze at a higher temperature. b. the great salt lake would freeze at a higher temperature. c. both lakes would freeze at the same temperature.
Answers: 2
You know the right answer?
This reaction is carried out at a different temperature with initial concentrations of [CO]=0.27M an...
Questions

English, 27.10.2020 19:10

Mathematics, 27.10.2020 19:10

Mathematics, 27.10.2020 19:10

Mathematics, 27.10.2020 19:10



Mathematics, 27.10.2020 19:10


Mathematics, 27.10.2020 19:10


Mathematics, 27.10.2020 19:10

History, 27.10.2020 19:10

Mathematics, 27.10.2020 19:10


Chemistry, 27.10.2020 19:10

Arts, 27.10.2020 19:10




History, 27.10.2020 19:10

![[H_2]](/tpl/images/0548/7512/08a38.png) =0.49M.
=0.49M. ![[CH_3OH]=0.11 M](/tpl/images/0548/7512/d5979.png)
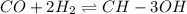
![K_c=\frac{[CH_3OH]}{[CO][H_2]^2}](/tpl/images/0548/7512/4cf94.png)




