
Chemistry, 16.03.2020 18:08 jasminer257
N unknown weak acid, HA, it titrated with 0.6 M NaOH. The pH at the halfway point of this titration was found to be 4.215. If the initial pH of the weak acid solution (before titration) has a pH of 2.148, what was the concentration of the weak acid solution

Answers: 2
Another question on Chemistry

Chemistry, 21.06.2019 13:50
How do the energy differences between the higher energy levels of an atom compare with the energy difference between the lower energy level of the atom
Answers: 1

Chemistry, 22.06.2019 14:50
Consider the following multistep reaction: a b→ab(slow) a ab→a2b(fast)−−−−−−−−−−−−−−−−− 2a b→a2b(overall) based on this mechanism, determine the rate law for the overall reaction. express your answer in standard masteringchemistry format. for example, if the rate law is k[a]3[b]2 type k*[a]^3*[b]^2
Answers: 3

Chemistry, 22.06.2019 21:00
What is the chemical formula for the compound formed between sodium and flour one
Answers: 1

Chemistry, 23.06.2019 00:30
What is bromine+calcium iodide--> calcium bromide +iodine balanced
Answers: 1
You know the right answer?
N unknown weak acid, HA, it titrated with 0.6 M NaOH. The pH at the halfway point of this titration...
Questions


Biology, 11.02.2021 01:00

Mathematics, 11.02.2021 01:00



Mathematics, 11.02.2021 01:00


English, 11.02.2021 01:00

Mathematics, 11.02.2021 01:00

Geography, 11.02.2021 01:00




Mathematics, 11.02.2021 01:00



Biology, 11.02.2021 01:00


Mathematics, 11.02.2021 01:00

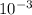 M
M



