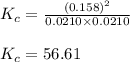Chemistry, 16.03.2020 16:29 jennemylesp19oy5
Suppose that 0.1000 mole each of H2and I2are placed in a 1.000-L flask, stoppered, and the mixture is heated to 425oC. At equilibrium, the concentration of I2is found to be 0.0210 M. Calculate Kcfor the following reaction at 425oC. H2(g) + I2(g) ⇄2 HI(g)

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 10:50
Someone offer some answers to this, i will give 98 coins and mark as brainliest! i will put the rest of the lab down in the comments,solutions pre-lab questions: in this lab, you will make fruit drinks with powdered drink mix. complete the pre-lab questions to get the values you need for your drink solutions. calculate the molar mass of powered fruit drink mix, made from sucrose (c12h22o11).using stoichiometry, determine the mass of powdered drink mix needed to make a 1.0 m solution of 100 ml. (hint: use molarity = to find the moles of drink mix, then convert moles to grams using a mole conversion.)what mass of powdered drink mix is needed to make a 0.5 m solution of 100 ml?
Answers: 1

Chemistry, 22.06.2019 14:30
For the reaction shown, find the limiting reactant for each of the following initial amounts of reactants. 4al(s)+3o2(g)→2al2o3(s) a) 1 molal, 1 mol o2 b) 4 molal, 2.6 mol o2 c) 16 molal, 13 mol o2 d) 7.4 molal, 6.5 mol o2
Answers: 3

Chemistry, 23.06.2019 06:00
What volume of 0.500 mol/l hydrochloric acid, hci (aq) is required to react completely with 1.00 g of aluminum hydroxide, ai(oh)3 (s)?
Answers: 1

Chemistry, 23.06.2019 12:10
Which structure is a valid representation of a hydrocarbon molecule?
Answers: 2
You know the right answer?
Suppose that 0.1000 mole each of H2and I2are placed in a 1.000-L flask, stoppered, and the mixture i...
Questions

English, 14.08.2021 14:00


English, 14.08.2021 14:00

Mathematics, 14.08.2021 14:00

English, 14.08.2021 14:00

English, 14.08.2021 14:00

Social Studies, 14.08.2021 14:00

Social Studies, 14.08.2021 14:00


Chemistry, 14.08.2021 14:00

World Languages, 14.08.2021 14:00



Geography, 14.08.2021 14:00

English, 14.08.2021 14:00

Mathematics, 14.08.2021 14:00

English, 14.08.2021 14:00

Computers and Technology, 14.08.2021 14:00

Computers and Technology, 14.08.2021 14:00

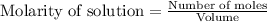
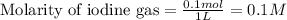
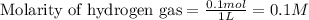
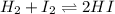
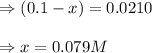
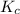 for above equation follows:
for above equation follows:![K_c=\frac{[HI]^2}{[H_2][I_2]}](/tpl/images/0548/4996/62646.png)
![[HI]_{eq}=2x=(2\times 0.079)=0.158M](/tpl/images/0548/4996/cad3c.png)
![[H_2]_{eq}=(0.1-x)=(0.1-0.079)=0.0210M](/tpl/images/0548/4996/029c8.png)
![[I_2]_{eq}=0.0210M](/tpl/images/0548/4996/0be20.png)